Basic Information
| Drug ID | DDPD01242 |

|
| Drug Name | Clomipramine | |
| Molecular Weight | 314.852 | |
| Molecular Formula | C19H23ClN2 | |
| CAS Number | 303-49-1 | |
| SMILES | CN(C)CCCN1C2=CC=CC=C2CCC2=C1C=C(Cl)C=C2 | |
| External Links | ||
| DRUGBANK | DB01242 | |
| T3DB | T3D3032 | |
| PubChem Compound | 2801 | |
| PDR | 678 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
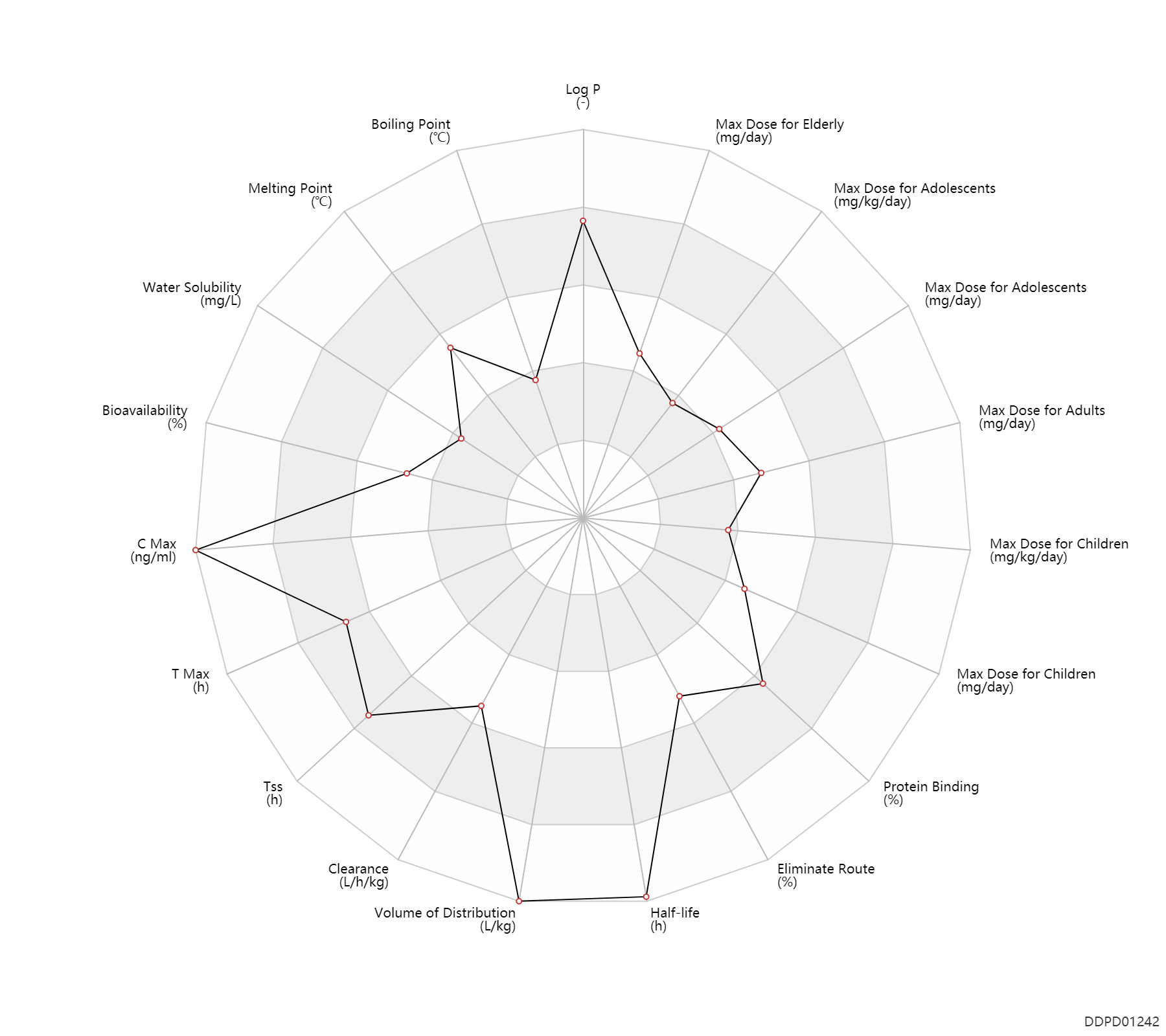
| Log P | 5.19 | - | 5.19 | - | HANSCH,C ET AL. (1995) |
| Boiling Point | 165.0 | ℃ | 160-170 | ℃ | PhysProp |
| Melting Point | 191.75 | ℃ | 191.5-192 | ℃ | Schindler, W. and Dietrich, H.; US. Patent 3,515,785; June 2,1970; assigned to Geigy Chemical Corp. |
| Water Solubility | 0.294 | mg/L | 0.294 | mg/L | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 50.0 | % | 50 | % | PO, oral; food; | food → ; | DRUGBANK |
| C Max | 92000000.0 | ng/ml | 92(56-154) | mg/ml | Oral single dose; | DRUGBANK | |
| T Max | 4.0 | h | 2-6 | h | Oral single dose; | DRUGBANK | |
| Tss | 252.0 | h | 1-2 | week | Oral multiple dose; | DRUGBANK | |
| Clearance | 0.49 | L/h/kg | 8.2 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset | |
| Volume of Distribution | 17.0 | L/kg | ~17(9-25) | L/kg | DRUGBANK | Volume of Distribution | 13.0 | L/kg | 13 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 32.0 | h | 32(19-37) | h | elimination half-life; Oral single dose; | DRUGBANK | Half-life | 69.0 | h | 69(54-77) | h | DRUGBANK | Half-life | 26.0 | h | 26 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 55.5 | % | 51-60 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 28.0 | % | 24-32 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 97.5 | % | ~97-98 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 3.0 | mg/kg/day | 3 | mg/kg/day | PO, oral | Anafranil | clomipramine hydrochloride | PDR |
| Max dose for children | 200.0 | mg/day | 200 | mg/day | PO, oral | Anafranil | clomipramine hydrochloride | PDR |
| Max dose for adolescents | 3.0 | mg/kg/day | 3 | mg/kg/day | PO, oral | Anafranil | clomipramine hydrochloride | PDR |
| Max dose for adolescents | 200.0 | mg/day | 200 | mg/day | PO, oral | Anafranil | clomipramine hydrochloride | PDR |
| Max dose for adults | 250.0 | mg/day | 250 | mg/day | PO, oral | Anafranil | clomipramine hydrochloride | PDR |
| Max dose for elderly | 250.0 | mg/day | 250 | mg/day | PO, oral | Anafranil | clomipramine hydrochloride | PDR |