Basic Information
| Drug ID | DDPD01238 |

|
| Drug Name | Aripiprazole | |
| Molecular Weight | 448.385 | |
| Molecular Formula | C23H27Cl2N3O2 | |
| CAS Number | 129722-12-9 | |
| SMILES | ClC1=CC=CC(N2CCN(CCCCOC3=CC4=C(CCC(=O)N4)C=C3)CC2)=C1Cl | |
| External Links | ||
| DRUGBANK | DB01238 | |
| T3DB | T3D2677 | |
| PubChem Compound | 60795 | |
| PDR | 103 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
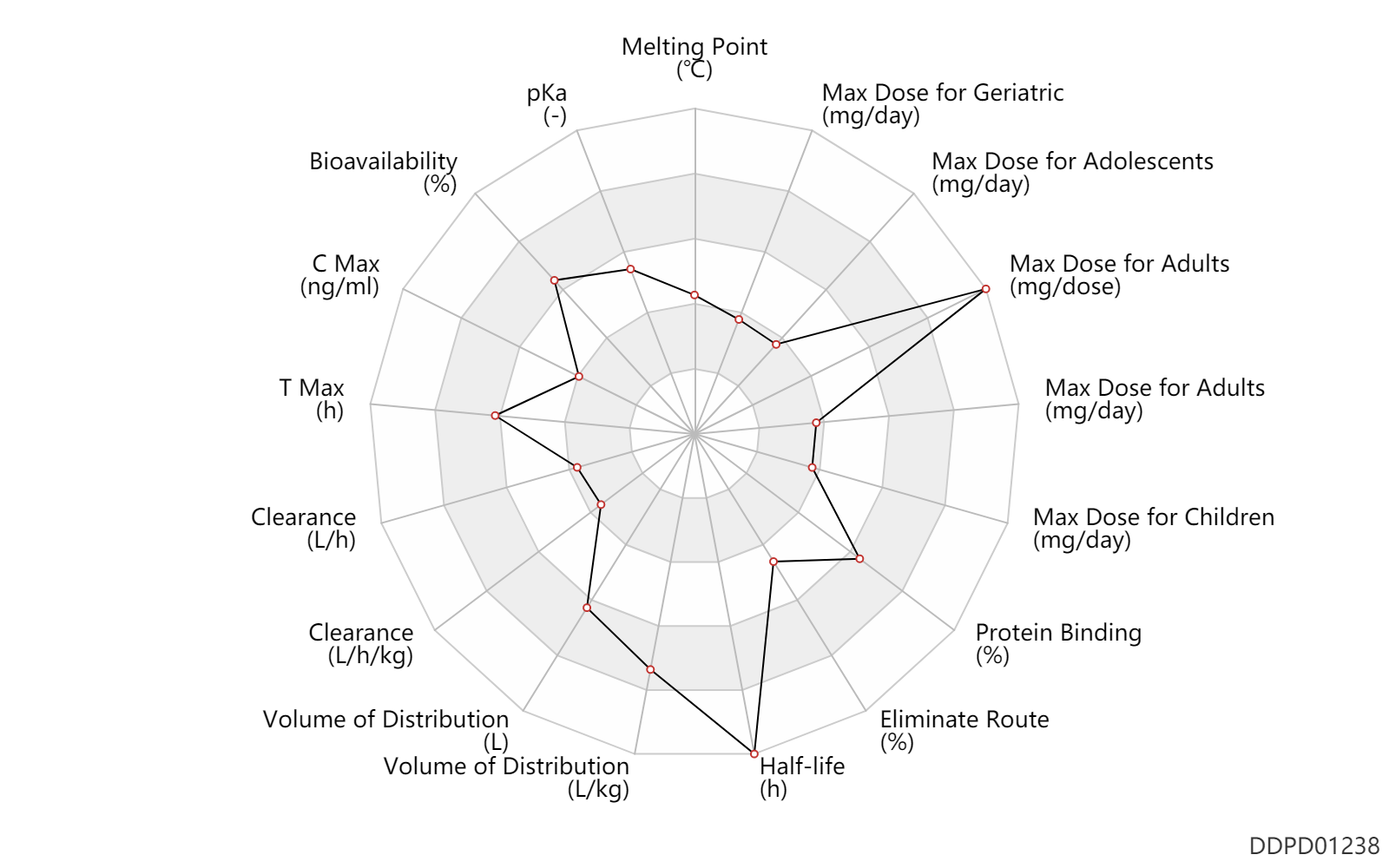
| Melting Point | 138.5 | ℃ | 137-140 | ℃ | http://www.chemspider.com/Chemical-Structure.54790.html |
| Water Solubility | 0.0 | % | 0 | % | https://medsafe.govt.nz/profs/Datasheet/a/Abilifytab.pdf |
| pKa | 7.6 | - | 7.6 | - | https://medsafe.govt.nz/profs/Datasheet/a/Abilifytab.pdf |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 87.0 | % | 87 | % | Tablet, PO, oral; | DRUGBANK | Bioavailability | 87.0 | % | 87.0 | % | PO, oral; | The Pharmacological Basis of Therapeutics |
| C Max | 242.0 | ng/ml | 242±36 | ng/ml | PO, oral; | The Pharmacological Basis of Therapeutics |
| T Max | 4.0 | h | 3-5 | h | Tablet, PO, oral; | DRUGBANK | T Max | 3.0 | h | 3.0±0.6 | h | PO, oral; | The Pharmacological Basis of Therapeutics |
| Clearance | 0.0480 | L/h/kg | 0.8 | ml/min/kg | DRUGBANK | Clearance | 3.3 | L/h | 3297±1042 | ml/h | DRUGBANK | Clearance | 0.0498 | L/h/kg | 0.83±0.17 | ml/min/kg | apparent clearance; at steady state; hydrolysis; hydrolysis; | The Pharmacological Basis of Therapeutics | Clearance | 0.0498 | L/h/kg | 0.83 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 404.0 | L | 404.0 | L | DRUGBANK | Volume of Distribution | 4.9 | L/kg | 4.9 | L/kg | Apparent volume of distribution; at steady state; hydrolysis; hydrolysis; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 4.9 | L/kg | 4.9 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 75.0 | h | 75 | h | DRUGBANK | Half-life | 94.0 | h | 94 | h | Active metabolite; | DRUGBANK | Half-life | 146.0 | h | 146 | h | poor CYP2D6 metabolize; | DRUGBANK | Half-life | 61.0 | h | 61.03±19.59 | h | different study; | DRUGBANK | Half-life | 279.0 | h | 279±299 | h | different study; Active metabolite; | DRUGBANK | Half-life | 47.0 | h | 47±10 | h | The Pharmacological Basis of Therapeutics | Half-life | 94.0 | h | 94.0 | h | Metabolite; | The Pharmacological Basis of Therapeutics | Half-life | 75.0 | h | 75 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 25.0 | % | 25 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 55.0 | % | 55 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 99.0 | % | >99 | % | DRUGBANK | Protein Binding | 99.0 | % | >99 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 30.0 | mg/day | 30 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 15.0 | mg/day | 15 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 30.0 | mg/day | 30 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 15.0 | mg/day | 15 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 10.0 | mg/day | 10 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 15.0 | mg/day | 15 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 20.0 | mg/day | 20 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 15.0 | mg/day | 15 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for children | 10.0 | mg/day | 10 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for adolescents | 30.0 | mg/day | 30 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for adolescents | 15.0 | mg/day | 15 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for adolescents | 20.0 | mg/day | 20 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for adolescents | 30.0 | mg/day | 30 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for adolescents | 15.0 | mg/day | 15 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for adolescents | 10.0 | mg/day | 10 | mg/day | PO, oral | Abilify | aripiprazole | PDR |
| Max dose for adults | 30.0 | mg/day | 30 | mg/day | Tablet,PO,oral | Abilify | aripiprazole | PDR |
| Max dose for adults | 25.0 | mg/day | 25 | mg/day | Liquid | Abilify | aripiprazole | PDR |
| Max dose for adults | 30.0 | mg/day | 30 | mg/day | IM,intramuscular injection | Abilify | aripiprazole | PDR |
| Max dose for adults | 13.3333333333333 | mg/day | 400 | mg/month | IM,intramuscular injection | Abilify | aripiprazole | PDR |
| Max dose for adults | 29.4 | mg/day | 882 | mg/month | IM,intramuscular injection | Abilify | aripiprazole | PDR |
| Max dose for adults | 675.0 | mg/dose | 675 | mg/dose | IM,intramuscular injection | Abilify | aripiprazole | PDR |
| Max dose for geriatric | 30.0 | mg/day | 30 | mg/day | Tablet,PO,oral | Abilify | aripiprazole | PDR |
| Max dose for geriatric | 25.0 | mg/day | 25 | mg/day | Liquid | Abilify | aripiprazole | PDR |
| Max dose for geriatric | 30.0 | mg/day | 30 | mg/day | IM,intramuscular injection | Abilify | aripiprazole | PDR |
| Max dose for geriatric | 13.3333333333333 | mg/day | 400 | mg/month | IM,intramuscular injection | Abilify | aripiprazole | PDR |
| Max dose for geriatric | 29.4 | mg/day | 882 | mg/month | IM,intramuscular injection | Abilify | aripiprazole | PDR |
| Max dose for geriatric | 675.0 | mg/dose | 675 | mg/dose | IM,intramuscular injection | Abilify | aripiprazole | PDR |