Basic Information
| Drug ID | DDPD01215 |

|
| Drug Name | Estazolam | |
| Molecular Weight | 294.738 | |
| Molecular Formula | C16H11ClN4 | |
| CAS Number | 29975-16-4 | |
| SMILES | ClC1=CC2=C(C=C1)N1C=NN=C1CN=C2C1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB01215 | |
| T3DB | T3D3023 | |
| PubChem Compound | 3261 | |
| PDR | 711 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
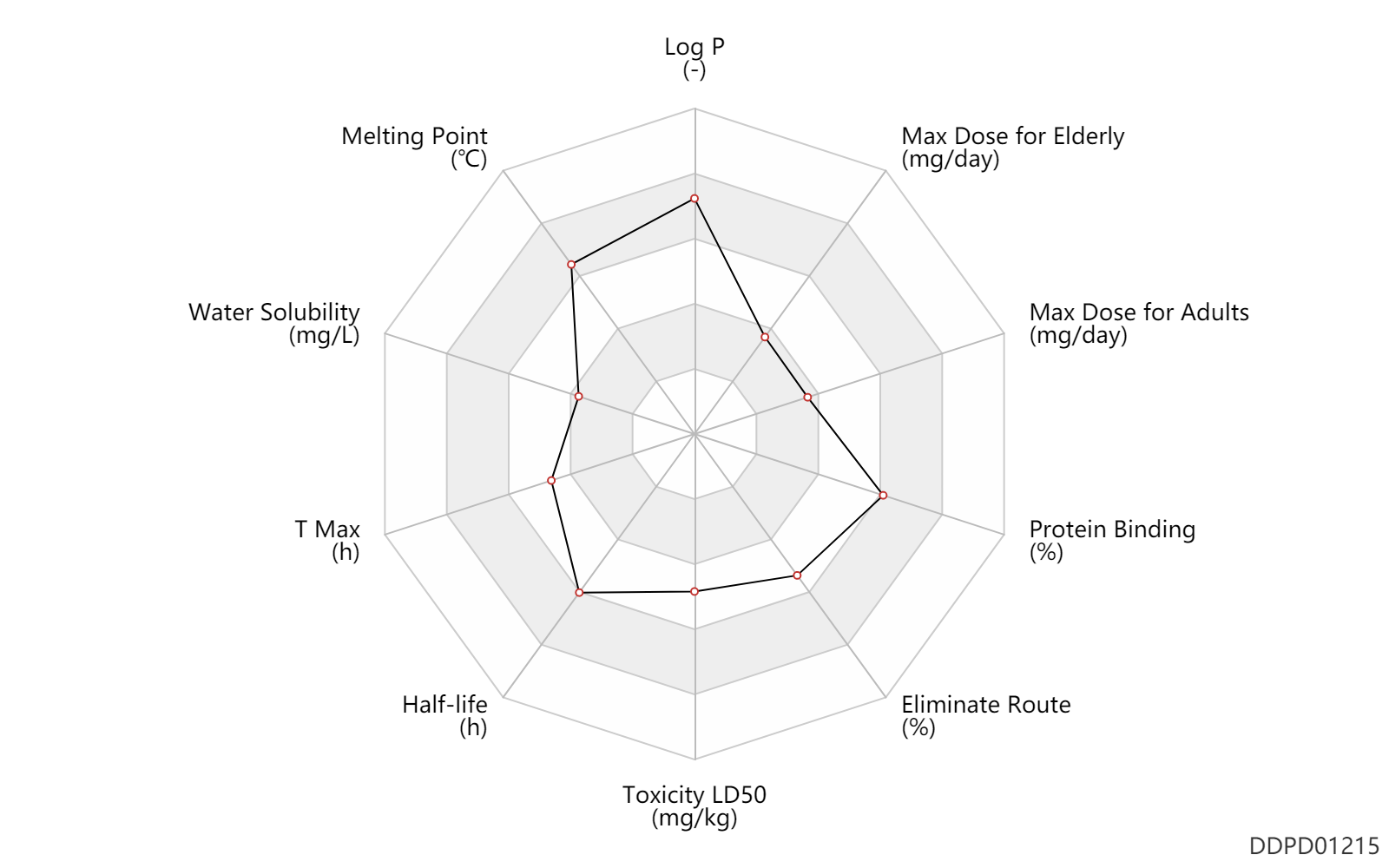
| Log P | 4.7 | - | 4.7 | - | DRUGBANK |
| Melting Point | 228.5 | ℃ | 228-229 | ℃ | Hester, J.B. Jr.; U.S. Patent 3,701,782; October 31, 1972; assigned to The Upjohn Co. |
| Water Solubility | 1.5 | mg/L | 1.5 | mg/L | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| T Max | 2.0 | h | 2(0.5-6) | h | PO, oral; | DRUGBANK | |
| Half-life | 17.0 | h | 10-24 | h | elimination half-life; | DRUGBANK | |
| Toxicity LD50 | 740.0 | mg/kg | 740.0 | mg/kg | PO, oral; male mouse; | T3DB | Toxicity LD50 | 3200.0 | mg/kg | 3200.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB | Toxicity LD50 | 300.0 | mg/kg | 300.0 | mg/kg | PO, oral; rabbit; | T3DB |
| Eliminate Route | 87.0 | % | 87 | % | Urinary excretion; human, homo sapiens; | DRUGBANK | Eliminate Route | 4.0 | % | <4 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 93.0 | % | 93 | % | Plasma Concentration → ; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 2.0 | mg/day | 2 | mg/day | PO, oral | Estazolam | estazolam | PDR |
| Max dose for elderly | 2.0 | mg/day | 2 | mg/day | PO, oral | Estazolam | estazolam | PDR |