Basic Information
| Drug ID | DDPD01203 |

|
| Drug Name | Nadolol | |
| Molecular Weight | 309.4006 | |
| Molecular Formula | C17H27NO4 | |
| CAS Number | 42200-33-9 | |
| SMILES | CC(C)(C)NCC(O)COC1=CC=CC2=C1C[C@H](O)[C@H](O)C2 | |
| External Links | ||
| DRUGBANK | DB01203 | |
| PubChem Compound | 39147 | |
| PDR | 1770 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
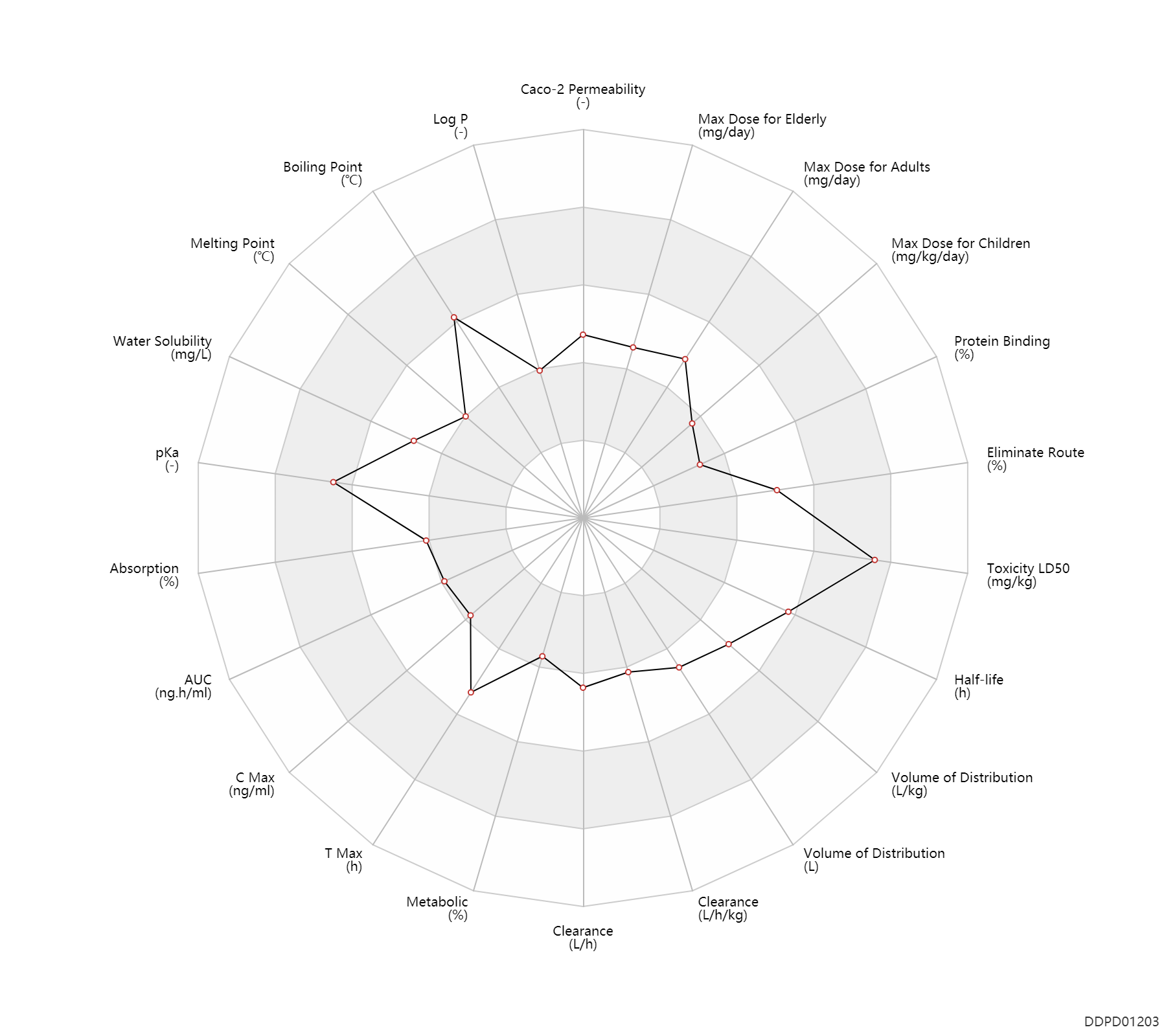
| Caco-2 Permeability | -5.41 | - | -5.41 | - | ADME Research, USCD |
| Log P | 0.81 | - | 0.81 | - | SANGSTER (1994) |
| Boiling Point | 526.437 | ℃ | 526.437 | ℃ | http://www.chemspider.com/Chemical-Structure.35815.html?rid=dffe98eb-8fd9-4c28-9a11-8dfe26bcaad3 |
| Melting Point | 127.0 | ℃ | 124-130 | ℃ | http://www.chemspider.com/Chemical-Structure.35815.html?rid=dffe98eb-8fd9-4c28-9a11-8dfe26bcaad3 |
| Water Solubility | 8330.0 | mg/L | 8330 | mg/L | MCFARLAND,JW ET AL. 2001) |
| pKa | 9.67 | - | 9.67 | - | MERCK INDEX (2001) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 30.0 | % | ~30 | % | PO, oral; | DRUGBANK |
| AUC | 1021.0 | ng.h/ml | 1021.0 | ng.h/ml | Oral single dose; | DRUGBANK | AUC | 1913.0 | ng.h/ml | 1913±382 | ng.h/ml | DRUGBANK |
| C Max | 69.0 | ng/ml | 69±15 | ng/ml | Oral single dose; | DRUGBANK | C Max | 132.0 | ng/ml | 132±27 | ng/ml | DRUGBANK |
| T Max | 2.7 | h | 2.7 | h | Oral single dose; | DRUGBANK |
| Metabolic | 0 | % | 0 | % | Liver metabolism; | DRUGBANK |
| Clearance | 14.1 | L/h | 219-250 | ml/min | Total clearance; normal,healthy; | DRUGBANK | Clearance | 8.4 | L/h | 131-150 | ml/min | Renal clearance; normal,healthy; | DRUGBANK | Clearance | 0.17 | L/h/kg | 2.9 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 152.0 | L | 147-157 | L | normal,healthy; | DRUGBANK | Volume of Distribution | 1.9 | L/kg | 1.9 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 22.0 | h | 20-24 | h | DRUGBANK | Half-life | 9.2 | h | 9.2 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 4500.0 | mg/kg | 4500.0 | mg/kg | PO, oral; mouse; | DRUGBANK |
| Eliminate Route | 60.0 | % | 60 | % | Urinary excretion; intravenous injection, IV; normal,healthy; human, homo sapiens; | DRUGBANK | Eliminate Route | 15.0 | % | 15 | % | Faeces excretion; intravenous injection, IV; normal,healthy; human, homo sapiens; | DRUGBANK |
| Protein Binding | 30.0 | % | ~30 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 2.5 | mg/kg/day | 2.5 | mg/kg/day | PO, oral | Corgard | nadolol | PDR |
| Max dose for adults | 320.0 | mg/day | 320 | mg/day | PO, oral | Corgard | nadolol | PDR |
| Max dose for adults | 240.0 | mg/day | 240 | mg/day | PO, oral | Corgard | nadolol | PDR |
| Max dose for elderly | 320.0 | mg/day | 320 | mg/day | PO, oral | Corgard | nadolol | PDR |
| Max dose for elderly | 240.0 | mg/day | 240 | mg/day | PO, oral | Corgard | nadolol | PDR |