Basic Information
| Drug ID | DDPD01196 |

|
| Drug Name | Estramustine | |
| Molecular Weight | 440.403 | |
| Molecular Formula | C23H31Cl2NO3 | |
| CAS Number | 2998-57-4 | |
| SMILES | [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])C3=CC=C(OC(=O)N(CCCl)CCCl)C=C3CC[C@@]21[H] | |
| External Links | ||
| DRUGBANK | DB01196 | |
| PubChem Compound | 259331 | |
| PDR | 1861 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
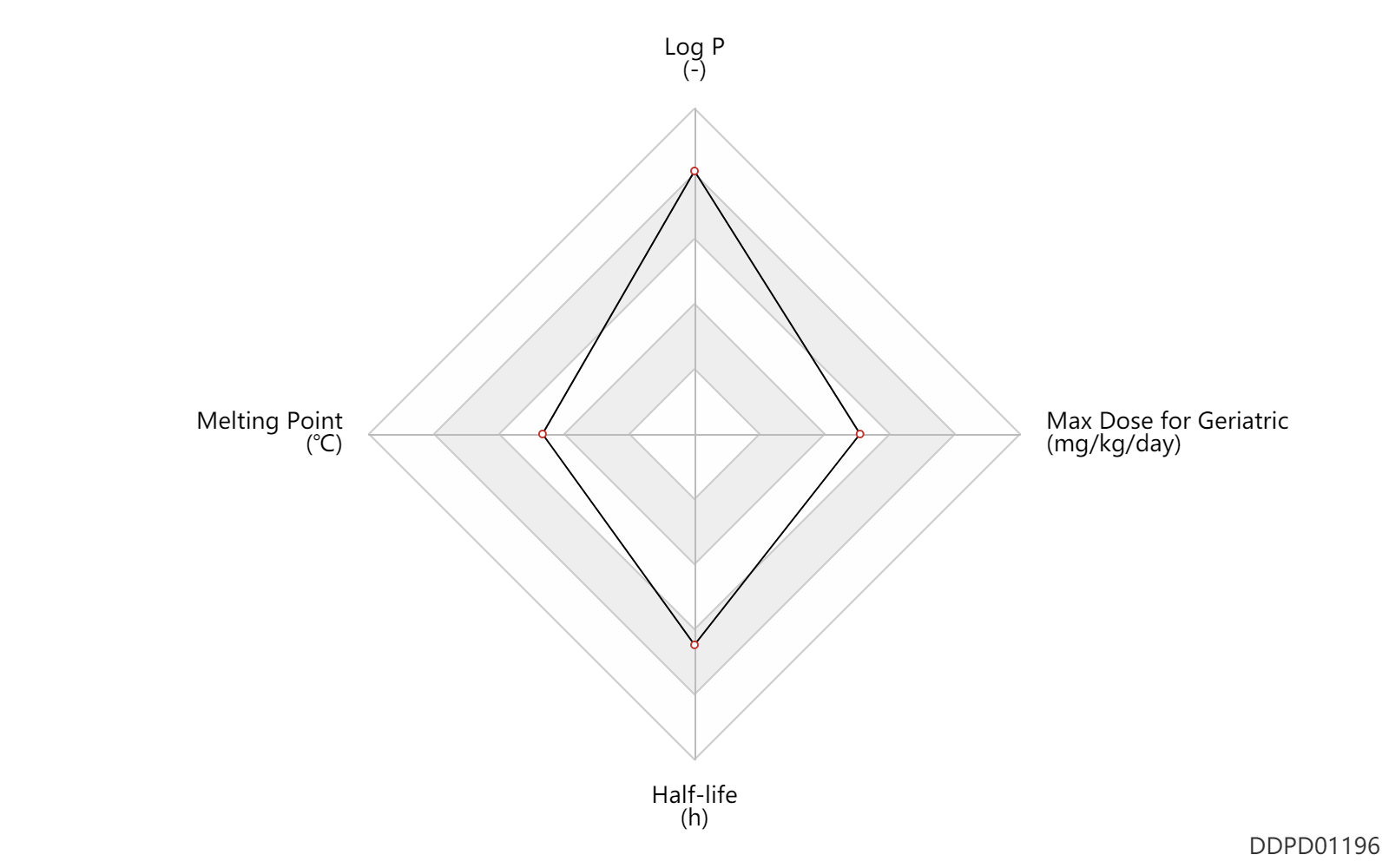
| Log P | 5.7 | - | 5.7 | - | DRUGBANK |
| Melting Point | 155.0 | ℃ | 155 | ℃ | Fex, H.J., Hogtierg, K.B., Konyves, I. and Kneip, P.H.0.L; U.S. Patent 3,299,104; Jan. 17, 1967; assigned to Leo AB, Sweden. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Half-life | 20.0 | h | 20 | h | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 16.0 | mg/kg/day | 16 | mg/kg/day | qd | Emcyt | estramustine phosphate sodium | PDR | |
| Max dose for geriatric | 16.0 | mg/kg/day | 16 | mg/kg/day | qd | Emcyt | estramustine phosphate sodium | PDR |