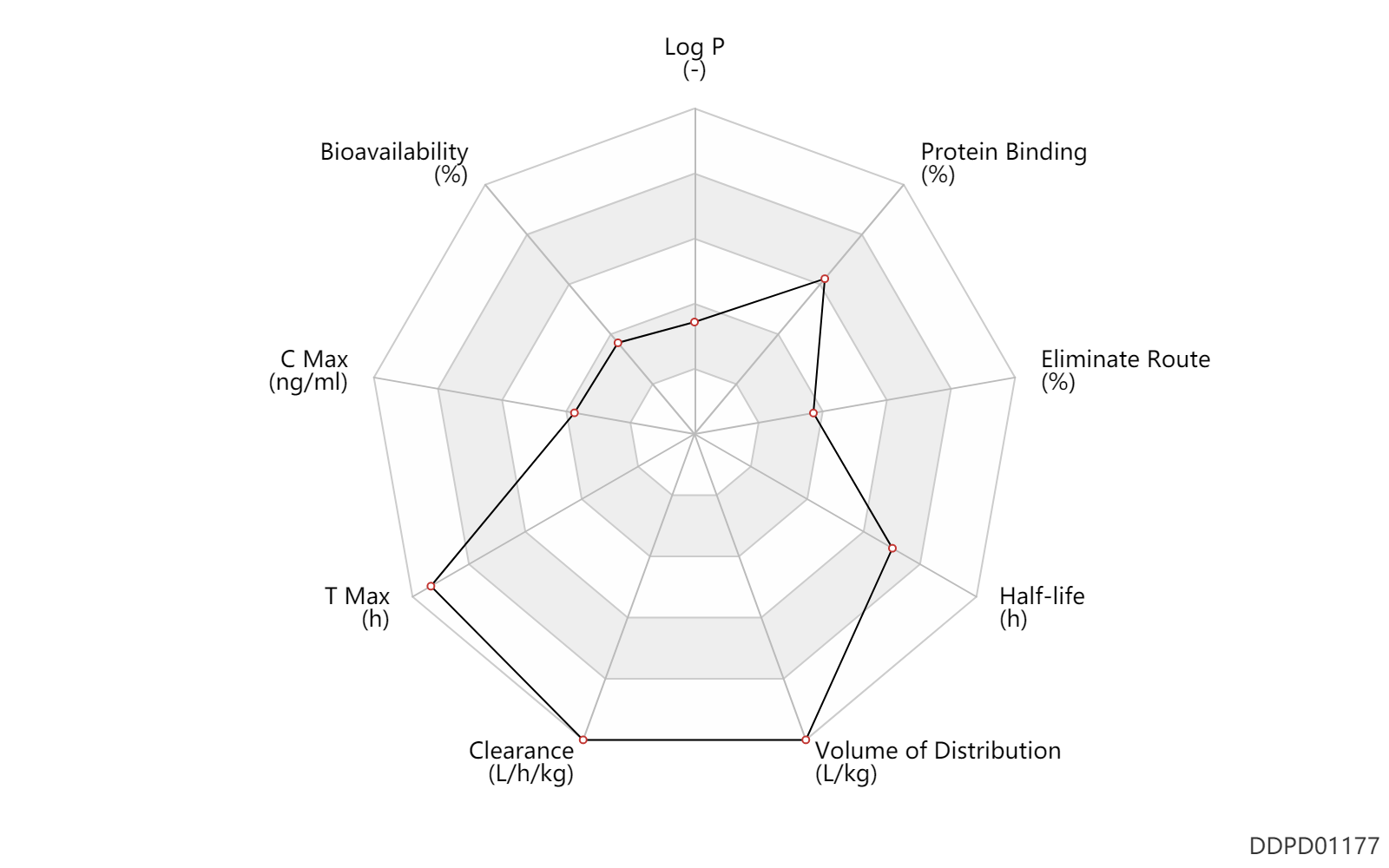
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Bioavailability |
28.0 |
% |
28±4 |
% |
PO, oral; tumor; |
|
The Pharmacological Basis of Therapeutics |
| C Max |
6.9 |
ng/ml |
6.9±0.1 |
ng/ml |
Oral single dose; tumor; |
|
The Pharmacological Basis of Therapeutics |
C Max |
22.0 |
ng/ml |
22±4 |
ng/ml |
Oral single dose; Active metabolite; tumor; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
5.4 |
h |
5.4±2.4 |
h |
Oral single dose; tumor; |
|
The Pharmacological Basis of Therapeutics |
T Max |
7.9 |
h |
7.9±2.3 |
h |
Oral single dose; Active metabolite; tumor; |
|
The Pharmacological Basis of Therapeutics |
| Clearance |
1.7 |
L/h/kg |
29±10 |
ml/min/kg |
|
mild renal function ↓ ;moderate renal function ↓ ; |
The Pharmacological Basis of Therapeutics |
Clearance |
1.4 |
L/h/kg |
24 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
24.7 |
L/kg |
24.7±5.9 |
L/kg |
|
|
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
38.0 |
L/kg |
38 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
22.0 |
h |
22 |
h |
|
|
DRUGBANK |
Half-life |
15.2 |
h |
15.2±3.7 |
h |
|
|
The Pharmacological Basis of Therapeutics |
Half-life |
41.0 |
h |
41±10 |
h |
|
mild renal function ↑ ;moderate renal function ↑ ; |
The Pharmacological Basis of Therapeutics |
Half-life |
16.0 |
h |
16 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route |
5.0 |
% |
<5 |
% |
Urinary excretion; tumor; human, homo sapiens; Unchanged drug; |
|
The Pharmacological Basis of Therapeutics |
| Protein Binding |
97.0 |
% |
97 |
% |
|
|
DRUGBANK |
Protein Binding |
97.0 |
% |
97 |
% |
tumor; human, homo sapiens; |
|
The Pharmacological Basis of Therapeutics |
Protein Binding |
94.0 |
% |
94 |
% |
Active metabolite; tumor; human, homo sapiens; |
|
The Pharmacological Basis of Therapeutics |