Basic Information
| Drug ID | DDPD01173 |

|
| Drug Name | Orphenadrine | |
| Molecular Weight | 269.3813 | |
| Molecular Formula | C18H23NO | |
| CAS Number | 83-98-7 | |
| SMILES | CN(C)CCOC(C1=CC=CC=C1)C1=CC=CC=C1C | |
| External Links | ||
| DRUGBANK | DB01173 | |
| T3DB | T3D3005 | |
| PubChem Compound | 4601 | |
| PDR | 1580 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
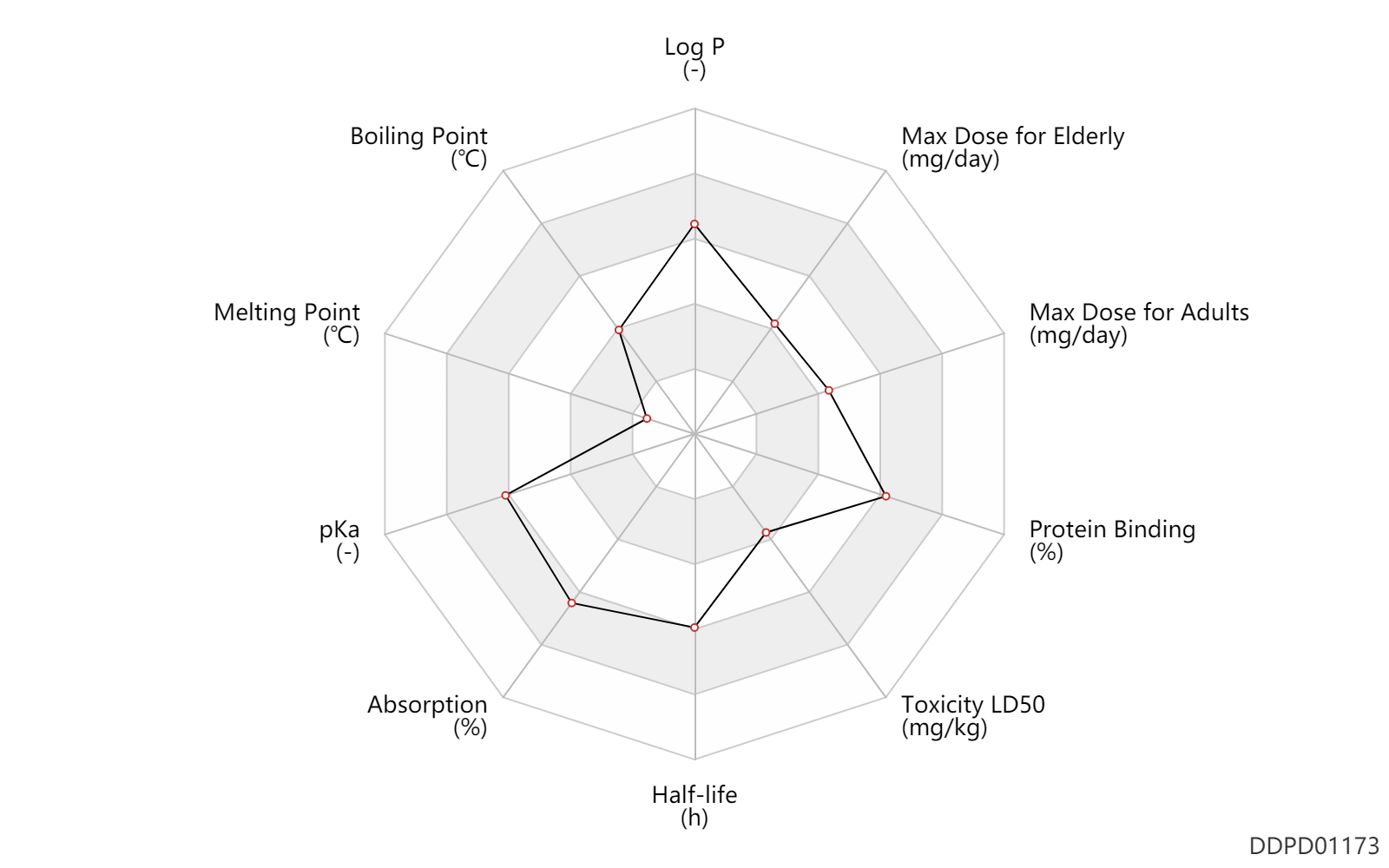
| Log P | 3.77 | - | 3.77 | - | SANGSTER (1993) |
| Boiling Point | 195.0 | ℃ | 195 | ℃ | PhysProp |
| Melting Point | 25.0 | ℃ | <25 | ℃ | PhysProp |
| pKa | 8.91 | - | 8.91 | - | SANGSTER (1994) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | ~100 | % | PO, oral; | DRUGBANK |
| Half-life | 16.5 | h | 13-20 | h | DRUGBANK | |
| Toxicity LD50 | 100.0 | mg/kg | 100.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 255.0 | mg/kg | 255.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 100.0 | mg/kg | 100.0 | mg/kg | PO, oral; mouse; | T3DB | Toxicity LD50 | 255.0 | mg/kg | 255.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB |
| Protein Binding | 95.0 | % | 95 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 200.0 | mg/day | 200 | mg/day | PO, oral | Orphenadrine Citrate Extended-Release Tablets | orphenadrine citrate | PDR |
| Max dose for adults | 120.0 | mg/day | 120 | mg/day | intravenous injection, IV;IM,intramuscular injection; | Orphenadrine Citrate Extended-Release Tablets | orphenadrine citrate | PDR |
| Max dose for elderly | 200.0 | mg/day | 200 | mg/day | PO, oral | Orphenadrine Citrate Extended-Release Tablets | orphenadrine citrate | PDR |
| Max dose for elderly | 120.0 | mg/day | 120 | mg/day | intravenous injection, IV;IM,intramuscular injection; | Orphenadrine Citrate Extended-Release Tablets | orphenadrine citrate | PDR |