Basic Information
| Drug ID | DDPD01119 |

|
| Drug Name | Diazoxide | |
| Molecular Weight | 230.671 | |
| Molecular Formula | C8H7ClN2O2S | |
| CAS Number | 364-98-7 | |
| SMILES | CC1=NS(=O)(=O)C2=C(N1)C=CC(Cl)=C2 | |
| External Links | ||
| DRUGBANK | DB01119 | |
| PubChem Compound | 3019 | |
| PDR | 565 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
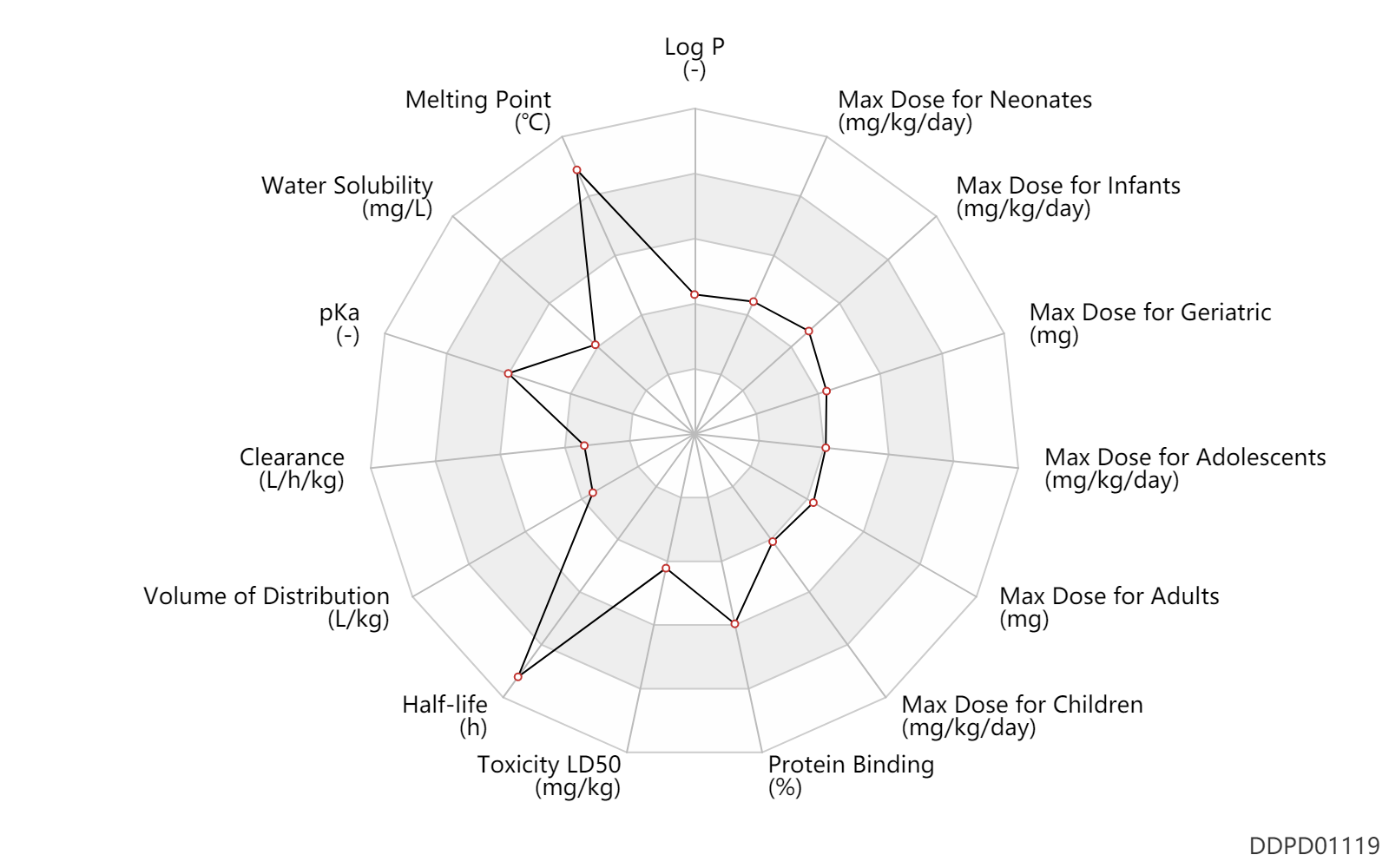
| Log P | 1.2 | - | 1.2 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 330.0 | ℃ | 330 | ℃ | Topliss, J.G., Sperber, N. and Rubin, A.A.; U.S. Patent 2,986,573; May 30, 1961; assigned to Schering Corporation. Topliss, J.G., Sperber, N. and Rubin, A.A.; U.S. Patent 3,345,365; October 3, 1967; assigned to Schering Corporation. |
| Water Solubility | 2850.0 | mg/L | 2850 | mg/L | DRUGBANK |
| pKa | 8.74 | - | 8.74 | - | SANGSTER (1994) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Clearance | 0.003600 | L/h/kg | 0.06 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.21 | L/kg | 0.21 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 28.0 | h | 28±8.3 | h | normal,healthy; adults; | DRUGBANK | Half-life | 48.0 | h | 48 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 980.0 | mg/kg | 980.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 444.0 | mg/kg | 444.0 | mg/kg | PO, oral; mouse; | DRUGBANK |
| Protein Binding | 90.0 | % | >90 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 20.0 | mg/kg/day | 20 | mg/kg/day | PO, oral | Proglycem | diazoxide | PDR |
| Max dose for infants | 20.0 | mg/kg/day | 20 | mg/kg/day | PO, oral | Proglycem | diazoxide | PDR |
| Max dose for children | 8.0 | mg/kg/day | 8 | mg/kg/day | PO, oral | Proglycem | diazoxide | PDR |
| Max dose for adolescents | 8.0 | mg/kg/day | 8 | mg/kg/day | PO, oral | Proglycem | diazoxide | PDR |
| Max dose for adults | 150.0 | mg | 150 | mg | intravenous injection, IV | Proglycem | diazoxide | PDR |
| Max dose for geriatric | 150.0 | mg | 150 | mg | intravenous injection, IV | Proglycem | diazoxide | PDR |