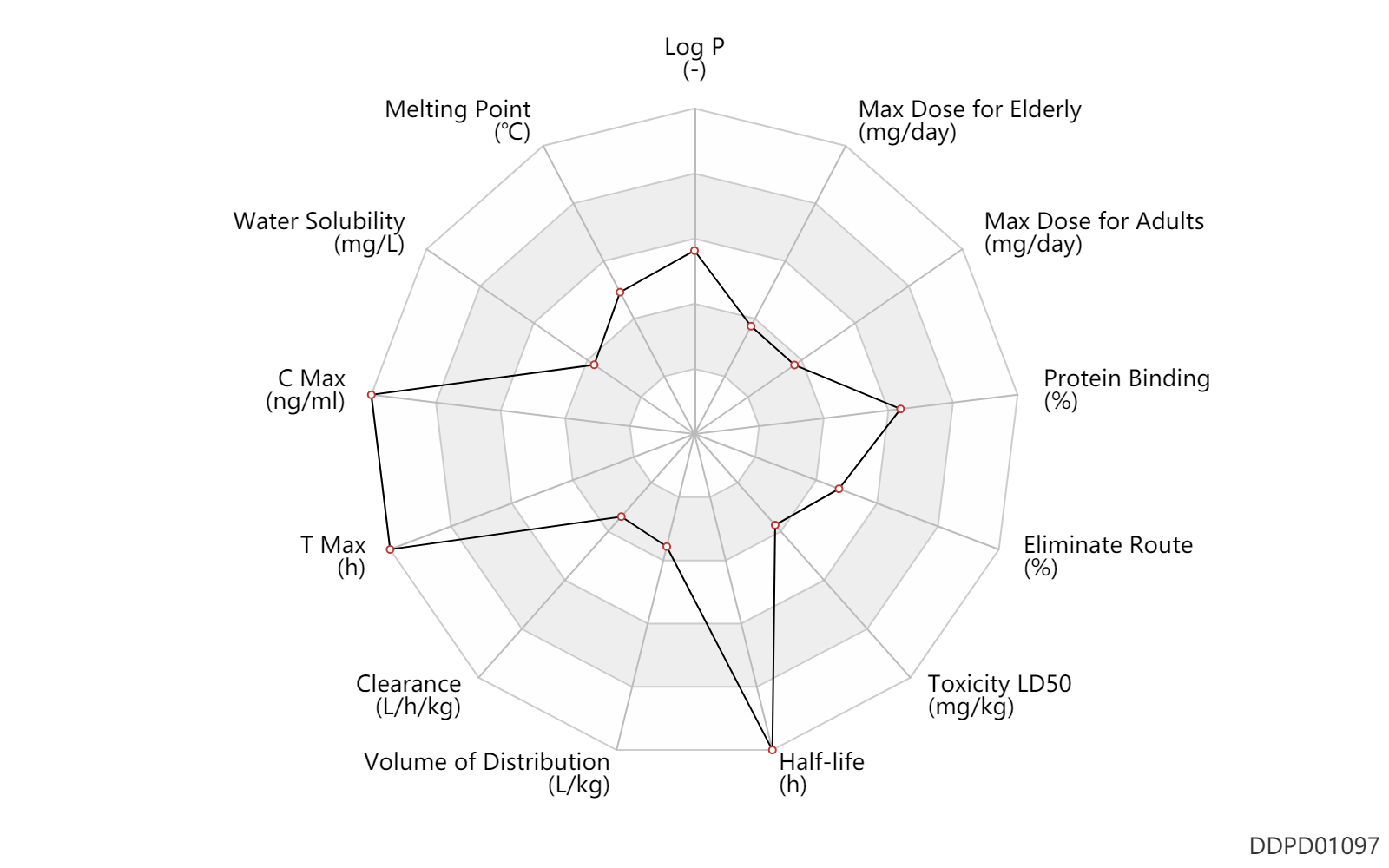
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| C Max |
35000.0 |
ng/ml |
35.0 |
ug/ml |
PO, oral; rheumatoid arthritis; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
9.0 |
h |
6-12 |
h |
|
|
DRUGBANK |
T Max |
9.0 |
h |
6-12 |
h |
PO, oral; rheumatoid arthritis; |
|
The Pharmacological Basis of Therapeutics |
| Clearance |
0.000720 |
L/h/kg |
0.012 |
ml/min/kg |
apparent clearance; normal,healthy; |
RD, renal impairment, Renal disease,including uremia ↑ ; |
The Pharmacological Basis of Therapeutics |
| Volume of Distribution |
0.13 |
L/kg |
0.13 |
L/kg |
|
|
DRUGBANK |
Volume of Distribution |
0.18 |
L/kg |
0.18(0.09-0.44) |
L/kg |
Apparent volume of distribution; normal,healthy; |
RD, renal impairment, Renal disease,including uremia ↑ ; |
The Pharmacological Basis of Therapeutics |
| Half-life |
336.0 |
h |
2 |
week |
|
|
DRUGBANK |
Half-life |
377.0 |
h |
377(336-432) |
h |
rheumatoid arthritis; patients; |
RD, renal impairment, Renal disease,including uremia → ; |
The Pharmacological Basis of Therapeutics |
| Toxicity LD50 |
175.0 |
mg/kg |
100-250 |
mg/kg |
PO, oral; |
|
DRUGBANK |
| Eliminate Route |
43.0 |
% |
~43 |
% |
Urinary excretion; Single dose; |
|
DRUGBANK |
Eliminate Route |
48.0 |
% |
~48 |
% |
Faeces excretion; Single dose; |
|
DRUGBANK |
Eliminate Route |
0 |
% |
~0 |
% |
Urinary excretion; Active metabolite; Unchanged drug; |
|
The Pharmacological Basis of Therapeutics |
| Protein Binding |
99.3 |
% |
>99.3 |
% |
|
|
DRUGBANK |
Protein Binding |
99.4 |
% |
99.4 |
% |
Active metabolite; |
RD, renal impairment, Renal disease,including uremia ↓ ; |
The Pharmacological Basis of Therapeutics |