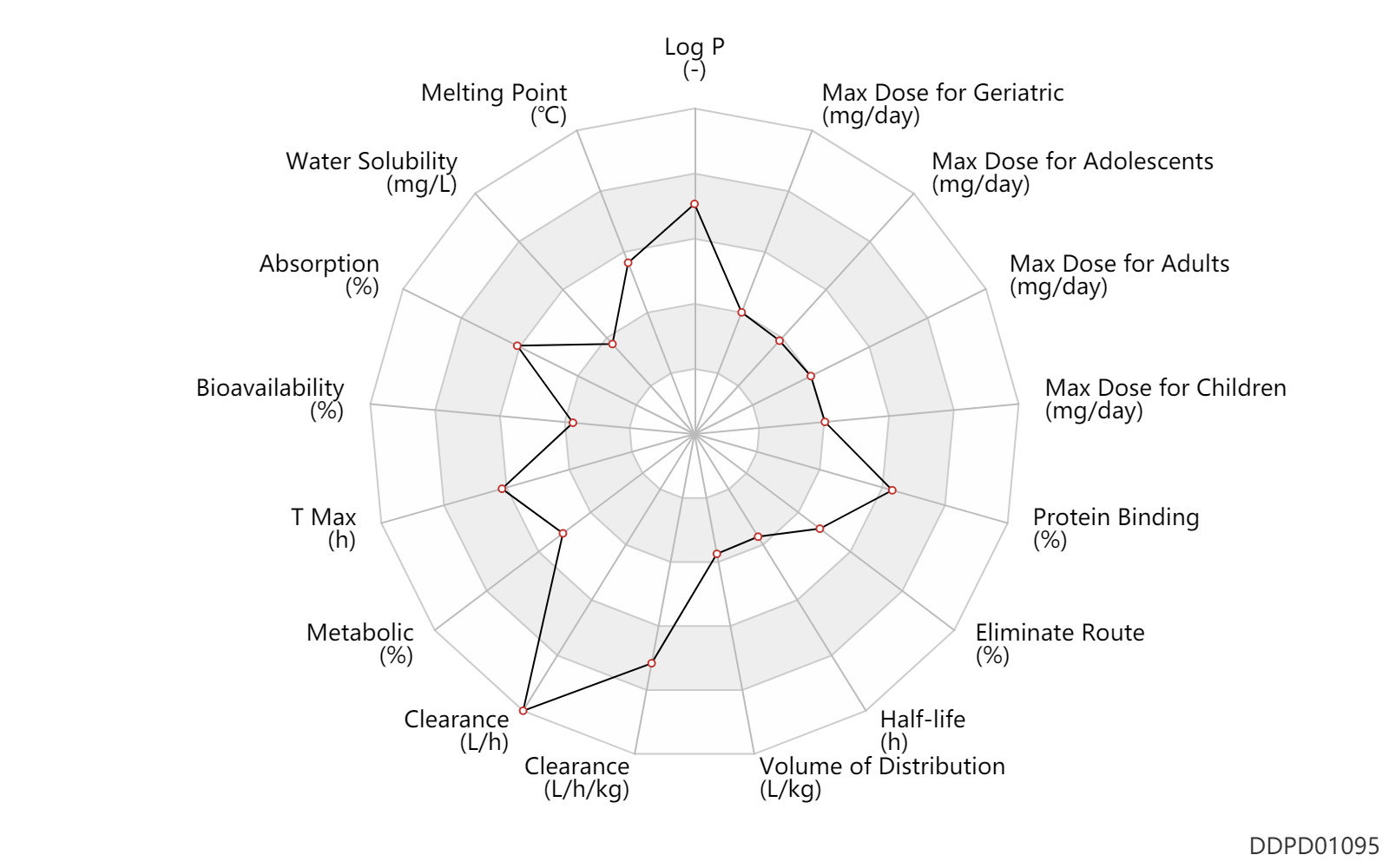
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Absorption |
90.0 |
% |
>90 |
% |
|
|
DRUGBANK |
| Bioavailability |
24.0 |
% |
24(9-50) |
% |
PO, oral; |
|
DRUGBANK |
Bioavailability |
36.0 |
% |
36 |
% |
PO, oral; extended release formulation; high-fat meal; |
extended release formulation ↑ ;high-fat meal ↑ ; |
DRUGBANK |
| T Max |
1.0 |
h |
<1 |
h |
PO, oral; |
|
DRUGBANK |
T Max |
6.0 |
h |
6 |
h |
PO, oral; extended release formulation; high-fat meal; |
extended release formulation ↑ ;high-fat meal ↑ ; |
DRUGBANK |
| Metabolic |
75.0 |
% |
75 |
% |
Liver metabolism; Faeces excretion; Bile excretion; |
|
DRUGBANK |
Metabolic |
20.0 |
% |
~20 |
% |
Liver metabolism; |
|
DRUGBANK |
Metabolic |
5.0 |
% |
~5 |
% |
Liver metabolism; |
|
DRUGBANK |
| Clearance |
0.80 |
L/h/kg |
0.8 |
L/h/kg |
|
|
DRUGBANK |
Clearance |
107.0 |
L/h |
107±38.1 |
L/h |
hypercholesterolemia; Single dose; |
|
DRUGBANK |
Clearance |
87.8 |
L/h |
87.8±45 |
L/h |
hypercholesterolemia; Multiple dose; |
|
DRUGBANK |
Clearance |
108.0 |
L/h |
108±44.7 |
L/h |
hypercholesterolemia; Single dose; |
|
DRUGBANK |
Clearance |
64.2 |
L/h |
64.2±21.1 |
L/h |
hypercholesterolemia; Multiple dose; |
|
DRUGBANK |
Clearance |
0.96 |
L/h/kg |
16 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
0.35 |
L/kg |
0.35 |
L/kg |
|
|
DRUGBANK |
Volume of Distribution |
0.42 |
L/kg |
0.42 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
3.0 |
h |
3 |
h |
|
|
DRUGBANK |
Half-life |
0.70 |
h |
0.7 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route |
90.0 |
% |
~90 |
% |
Faeces excretion; PO, oral; |
|
DRUGBANK |
Eliminate Route |
5.0 |
% |
~5 |
% |
Urinary excretion; PO, oral; |
|
DRUGBANK |
Eliminate Route |
1.8 |
% |
<1.8 |
% |
Faeces excretion; PO, oral; Unchanged drug; |
|
DRUGBANK |
| Protein Binding |
98.0 |
% |
98 |
% |
plasma proteins; |
|
DRUGBANK |