Basic Information
| Drug ID | DDPD01080 |

|
| Drug Name | Vigabatrin | |
| Molecular Weight | 129.157 | |
| Molecular Formula | C6H11NO2 | |
| CAS Number | 68506-86-5 | |
| SMILES | NC(CCC(O)=O)C=C | |
| External Links | ||
| DRUGBANK | DB01080 | |
| T3DB | T3D2977 | |
| PubChem Compound | 5665 | |
| PDR | 296 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
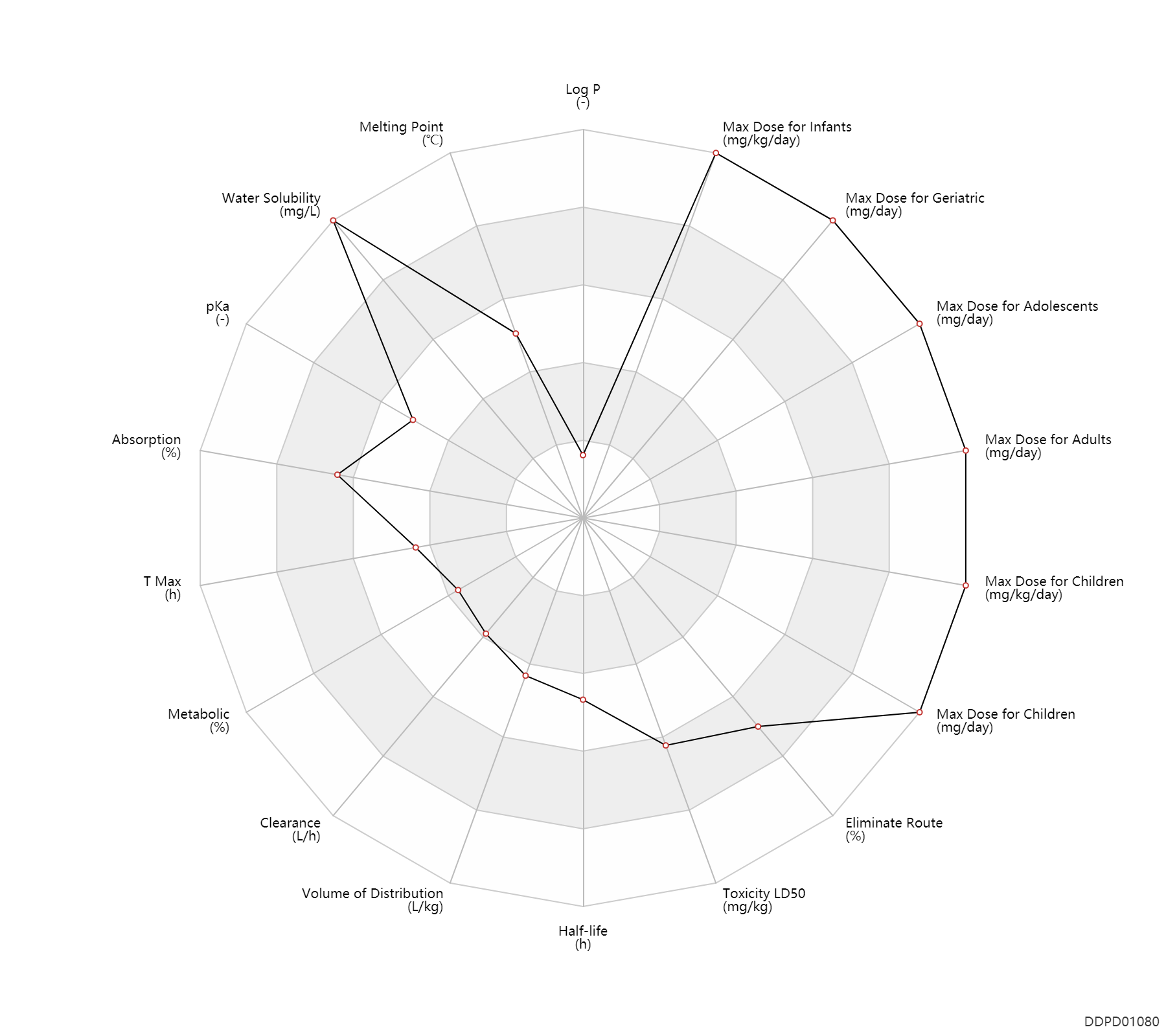
| Log P | -1.96 | - | -1.96 | - | FDA Label |
| Melting Point | 171.0 | ℃ | 171-176 | ℃ | FDA Label |
| Water Solubility | 55100.0 | mg/L | 55.1 | mg/ml | FDA Label |
| pKa | 4.0 | - | 4,9.7 | - | FDA Label | pKa | 9.7 | - | 4,9.7 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | ~100 | % | PO, oral; | DRUGBANK | |
| T Max | 1.0 | h | 1 | h | PO, oral; | DRUGBANK | T Max | 2.5 | h | 2.5 | h | PO, oral; Infants; | Infants ↑ ; | DRUGBANK |
| Metabolic | 0 | % | 0 | % | DRUGBANK | ||
| Clearance | 2.4 | L/h | 2.4 | L/h | PO, oral; Infants; | DRUGBANK | Clearance | 5.1 | L/h | 5.1 | L/h | PO, oral; Children; | DRUGBANK | Clearance | 5.8 | L/h | 5.8 | L/h | PO, oral; Adolescents; | DRUGBANK | Clearance | 7.0 | L/h | 7.0 | L/h | PO, oral; adults; | DRUGBANK |
| Volume of Distribution | 1.1 | L/kg | 1.1 | L/kg | Steady state volume of distribution; | DRUGBANK | |
| Half-life | 5.7 | h | ~5.7 | h | terminal half-life; Infants; | DRUGBANK | Half-life | 7.0 | h | 6-8 | h | terminal half-life; Children; | DRUGBANK | Half-life | 9.5 | h | 9.5 | h | terminal half-life; Adolescents; | DRUGBANK | Half-life | 10.5 | h | 10.5 | h | terminal half-life; adults; | DRUGBANK |
| Toxicity LD50 | 2830.0 | mg/kg | 2830.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 3100.0 | mg/kg | 3100.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 3000.0 | mg/kg | 3000.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB |
| Eliminate Route | 95.0 | % | ~95 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 76.0 | % | ~76 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 150.0 | mg/kg/day | 150 | mg/kg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for children | 3000.0 | mg/day | 3000 | mg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for children | 2000.0 | mg/day | 2000 | mg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for children | 150.0 | mg/kg/day | 150 | mg/kg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for children | 150.0 | mg/kg/day | 150 | mg/kg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for children | 150.0 | mg/kg/day | 150 | mg/kg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for adolescents | 3000.0 | mg/day | 3000 | mg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for adolescents | 3000.0 | mg/day | 3000 | mg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for adolescents | 2000.0 | mg/day | 2000 | mg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for adults | 3000.0 | mg/day | 3000 | mg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |
| Max dose for geriatric | 3000.0 | mg/day | 3000 | mg/day | PO, oral | Sabril for Oral Solution | vigabatrin | PDR |