Basic Information
| Drug ID | DDPD01075 |

|
| Drug Name | Diphenhydramine | |
| Molecular Weight | 255.3547 | |
| Molecular Formula | C17H21NO | |
| CAS Number | 58-73-1 | |
| SMILES | CN(C)CCOC(C1=CC=CC=C1)C1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB01075 | |
| T3DB | T3D2976 | |
| PubChem Compound | 3100 | |
| PDR | 1140 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
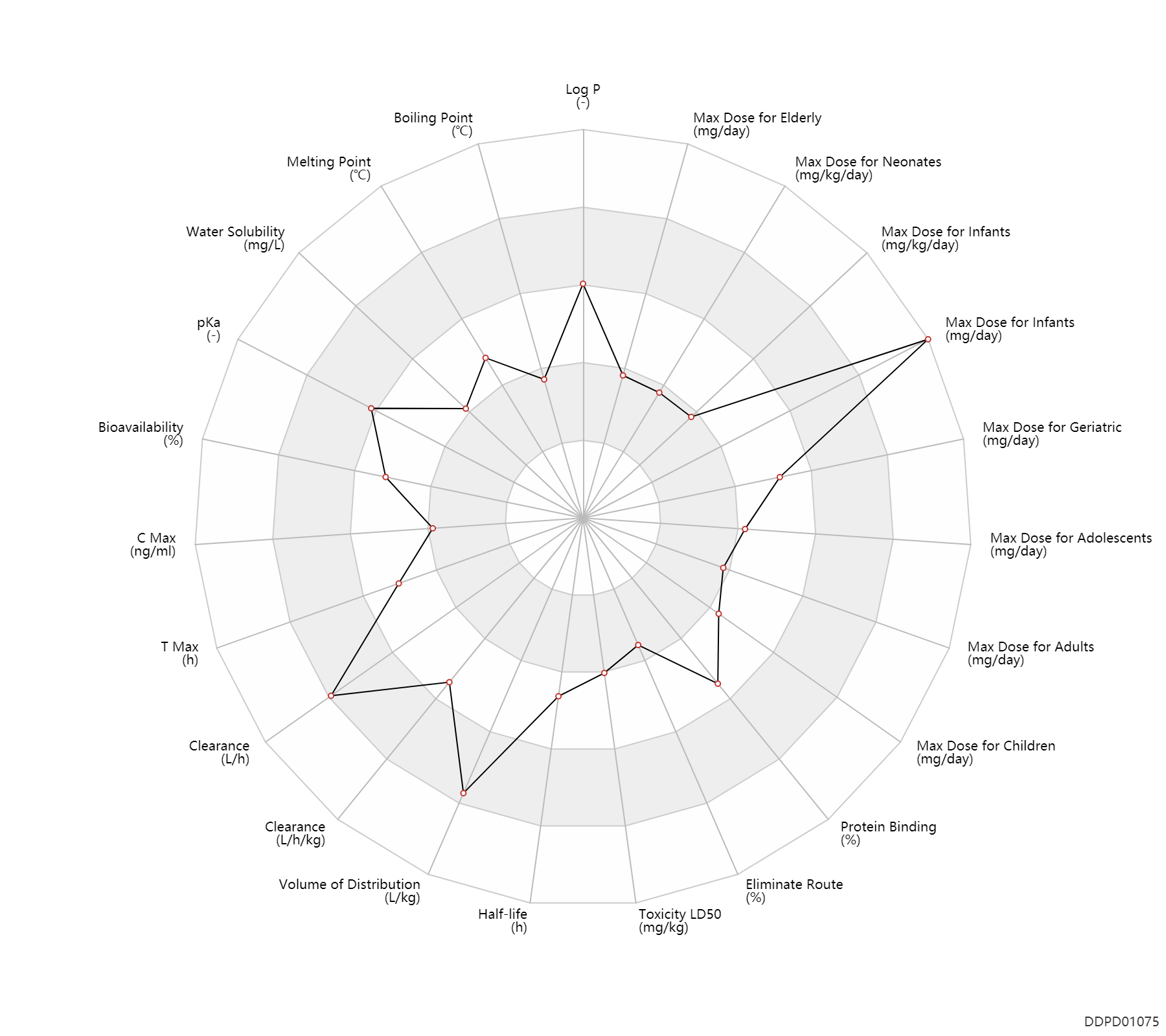
| Log P | 3.27 | - | 3.27 | - | HANSCH,C ET AL. (1995) |
| Boiling Point | 157.5 | ℃ | 150-165 | ℃ | PhysProp |
| Melting Point | 161.5 | ℃ | 161-162 | ℃ | Martin, H., Hafliger, F.,Gatzi, K.and Grob, A.; U.S. Patent 2,397,799; April 2,1946; assigned to J.R. Geigy AG, Switzerland. Rieveschl, G. Jr.; U.S. Patent 2,421,714; June 3,1947; assigned to Parke, Davis & Co. Rieveschl, G. Jr.; U.S. Patent 2,427,878; September 23,1947; assigned to Parke, Davis & Company. |
| Water Solubility | 3060.0 | mg/L | 3060 | mg/L | BEILSTEIN |
| pKa | 8.98 | - | 8.98 | - | SANGSTER (1994) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 50.0 | % | 40-60 | % | PO, oral; | DRUGBANK | Bioavailability | 72.0 | % | 72±26 | % | PO, oral; | The Pharmacological Basis of Therapeutics |
| C Max | 66.0 | ng/ml | 66±22 | ng/ml | Oral single dose; fasting; adults; normal,healthy; | The Pharmacological Basis of Therapeutics | C Max | 230.0 | ng/ml | ~230 | ng/ml | fasting; adults; normal,healthy; | The Pharmacological Basis of Therapeutics |
| T Max | 2.5 | h | 2-3 | h | PO, oral; | DRUGBANK | T Max | 2.3 | h | 2.3±0.64 | h | Oral single dose; fasting; adults; normal,healthy; | The Pharmacological Basis of Therapeutics |
| Clearance | 57.0 | L/h | 600-1300 | ml/min | Plasma clearance; PO, oral; | DRUGBANK | Clearance | 0.37 | L/h/kg | 6.2±1.7 | ml/min/kg | Children ↑ ;Elderly ↓ ;Asian ↑ ;Hepatic cirrhosis, cirr → ; | The Pharmacological Basis of Therapeutics | Clearance | 0.59 | L/h/kg | 9.8 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 5.1 | L/kg | 3.3-6.8 | L/kg | PO, oral; | DRUGBANK | Volume of Distribution | 4.5 | L/kg | 4.5±2.8 | L/kg | Apparent volume of distribution; | Asian ↓ ;Hepatic cirrhosis, cirr → ; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 6.5 | L/kg | 6.5 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 5.9 | h | 2.4-9.3 | h | elimination half-life; normal,healthy; adults; | Hepatitis, Hep ↑ ; | DRUGBANK | Half-life | 8.5 | h | 8.5±3.2 | h | Asian → ;Children ↓ ;Hepatic cirrhosis, cirr ↑ ;Age ↑ ; | The Pharmacological Basis of Therapeutics | Half-life | 9.3 | h | 9.3 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 500.0 | mg/kg | 500.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 500.0 | mg/kg | 500.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB |
| Eliminate Route | 1.0 | % | ~1 | % | Urinary excretion; Single dose; Unchanged drug; | DRUGBANK | Eliminate Route | 1.9 | % | 1.9±0.8 | % | Urinary excretion; Unchanged drug; | Hepatic cirrhosis, cirr → ; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 78.0 | % | ~78 | % | DRUGBANK | Protein Binding | 82.5 | % | 80-85 | % | plasma proteins; | DRUGBANK | Protein Binding | 78.0 | % | 78±3 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 5.0 | mg/kg/day | 5 | mg/kg/day | intravenous injection, IV;IM,intramuscular injection; | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for infants | 5.0 | mg/kg/day | 5 | mg/kg/day | PO, oral;intravenous injection, IV;IM,intramuscular injection; | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for infants | 300.0 | mg/day | 300 | mg/day | PO, oral;intravenous injection, IV;IM,intramuscular injection; | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for children | 300.0 | mg/day | 300 | mg/day | PO, oral | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for children | 400.0 | mg/day | 400 | mg/day | intravenous injection, IV;IM,intramuscular injection; | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for adolescents | 300.0 | mg/day | 300 | mg/day | PO, oral | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for adolescents | 400.0 | mg/day | 400 | mg/day | intravenous injection, IV;IM,intramuscular injection; | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for geriatric | 300.0 | mg/day | 300 | mg/day | PO, oral | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for geriatric | 400.0 | mg/day | 400 | mg/day | intravenous injection, IV;IM,intramuscular injection; | Diphenhydramine Hydrochloride | diphenhydramine hydrochloride | PDR |
| Max dose for children | 25.0 | mg/day | 25 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for children | 60.0 | mg/day | 60 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for children | 30.0 | mg/day | 30 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for children | 15.0 | mg/day | 15 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for adolescents | 25.0 | mg/day | 25 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for adolescents | 60.0 | mg/day | 60 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for adults | 25.0 | mg/day | 25 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for adults | 60.0 | mg/day | 60 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for elderly | 25.0 | mg/day | 25 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |
| Max dose for elderly | 60.0 | mg/day | 60 | mg/day | PO, oral | Unisom | doxylamine succinate | PDR |