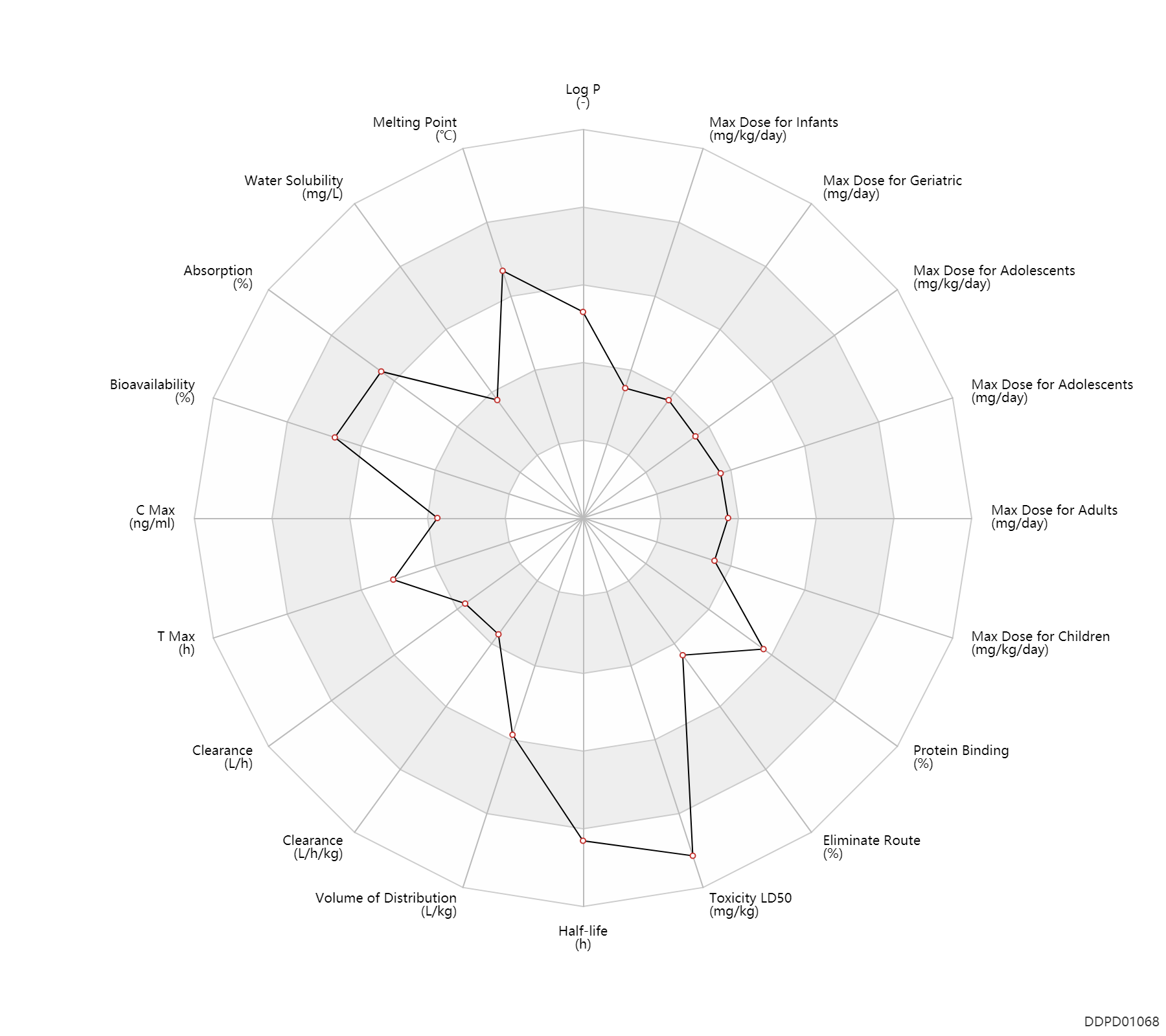
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Reference |
| Log P |
2.41 |
- |
2.41 |
- |
HANSCH,C ET AL. (1995) |
| Melting Point |
239.0 |
℃ |
238-240 |
℃ |
Kariss, J. and Newmark, H.L.; US. Patents 3,116,203; December 31, 1963; and 3,123,529; March 3, 1964; both assigned to Hoffmann-LaRoche, Inc.
Keller, O., Steiger, N. and Sternbach, L.H.; U S . Patents 3,121,114; February 11, 1964; and 3,203990; August 31, 1965; both assigned to Hoffmann-LaRoche, Inc.
Focella, A. and Rachlin, A.I.; U.S. Patent 3,335,181; August 8, 1967; assigned to Hoffmann- LaRoche. Inc. |
| Water Solubility |
100.0 |
mg/L |
100 |
mg/L |
MERCK INDEX 1996) |
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Absorption |
100.0 |
% |
~100 |
% |
Tablet, PO, oral; |
|
DRUGBANK |
| Bioavailability |
90.0 |
% |
~90 |
% |
Tablet, PO, oral; |
|
DRUGBANK |
Bioavailability |
98.0 |
% |
98±31 |
% |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| C Max |
17.0 |
ng/ml |
17±5.4 |
ng/ml |
Tablet, PO, oral; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
C Max |
16.0 |
ng/ml |
3-29 |
ng/ml |
intravenous injection, IV; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
2.5 |
h |
1-4 |
h |
Tablet, PO, oral; |
|
DRUGBANK |
T Max |
2.5 |
h |
2.5±1.3 |
h |
Tablet, PO, oral; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
| Clearance |
3.3 |
L/h |
~55 |
ml/min |
|
gaining weight ↓ ; |
DRUGBANK |
Clearance |
0.0930 |
L/h/kg |
1.55±0.28 |
ml/min/kg |
apparent clearance; hydrolysis; |
|
The Pharmacological Basis of Therapeutics |
Clearance |
0.0528 |
L/h/kg |
0.88 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
3.0 |
L/kg |
~3 |
L/kg |
Apparent volume of distribution; |
|
DRUGBANK |
Volume of Distribution |
3.2 |
L/kg |
3.2±1.1 |
L/kg |
|
|
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
2.9 |
L/kg |
2.9 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
35.0 |
h |
~30-40 |
h |
elimination half-life; |
dose → ; |
DRUGBANK |
Half-life |
23.0 |
h |
23±5 |
h |
|
|
The Pharmacological Basis of Therapeutics |
Half-life |
38.0 |
h |
38 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 |
4000.0 |
mg/kg |
4000.0 |
mg/kg |
PO, oral; mouse; |
|
DRUGBANK |
Toxicity LD50 |
4000.0 |
mg/kg |
4000.0 |
mg/kg |
PO, oral; adults; Rattus, Rat; |
|
DRUGBANK |
Toxicity LD50 |
2000.0 |
mg/kg |
2000.0 |
mg/kg |
PO, oral; rabbit; |
|
DRUGBANK |
Toxicity LD50 |
15000.0 |
mg/kg |
>15000 |
mg/kg |
PO, oral; Rattus, Rat; |
|
T3DB |
| Eliminate Route |
60.0 |
% |
~50-70 |
% |
Urinary excretion; |
|
DRUGBANK |
Eliminate Route |
2.0 |
% |
<2 |
% |
Urinary excretion; Unchanged drug; |
|
DRUGBANK |
Eliminate Route |
1.0 |
% |
<1 |
% |
Urinary excretion; Unchanged drug; |
|
The Pharmacological Basis of Therapeutics |
| Protein Binding |
84.0 |
% |
82-86 |
% |
plasma proteins; |
|
DRUGBANK |
Protein Binding |
86.0 |
% |
86±0.5 |
% |
|
Neonates ↓ ; |
The Pharmacological Basis of Therapeutics |