Basic Information
| Drug ID | DDPD01057 |

|
| Drug Name | Echothiophate | |
| Molecular Weight | 256.323 | |
| Molecular Formula | C9H23NO3PS | |
| CAS Number | 6736-03-4 | |
| SMILES | CCOP(=O)(OCC)SCC[N+](C)(C)C | |
| External Links | ||
| DRUGBANK | DB01057 | |
| PubChem Compound | 10548 | |
| PDR | 1953 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
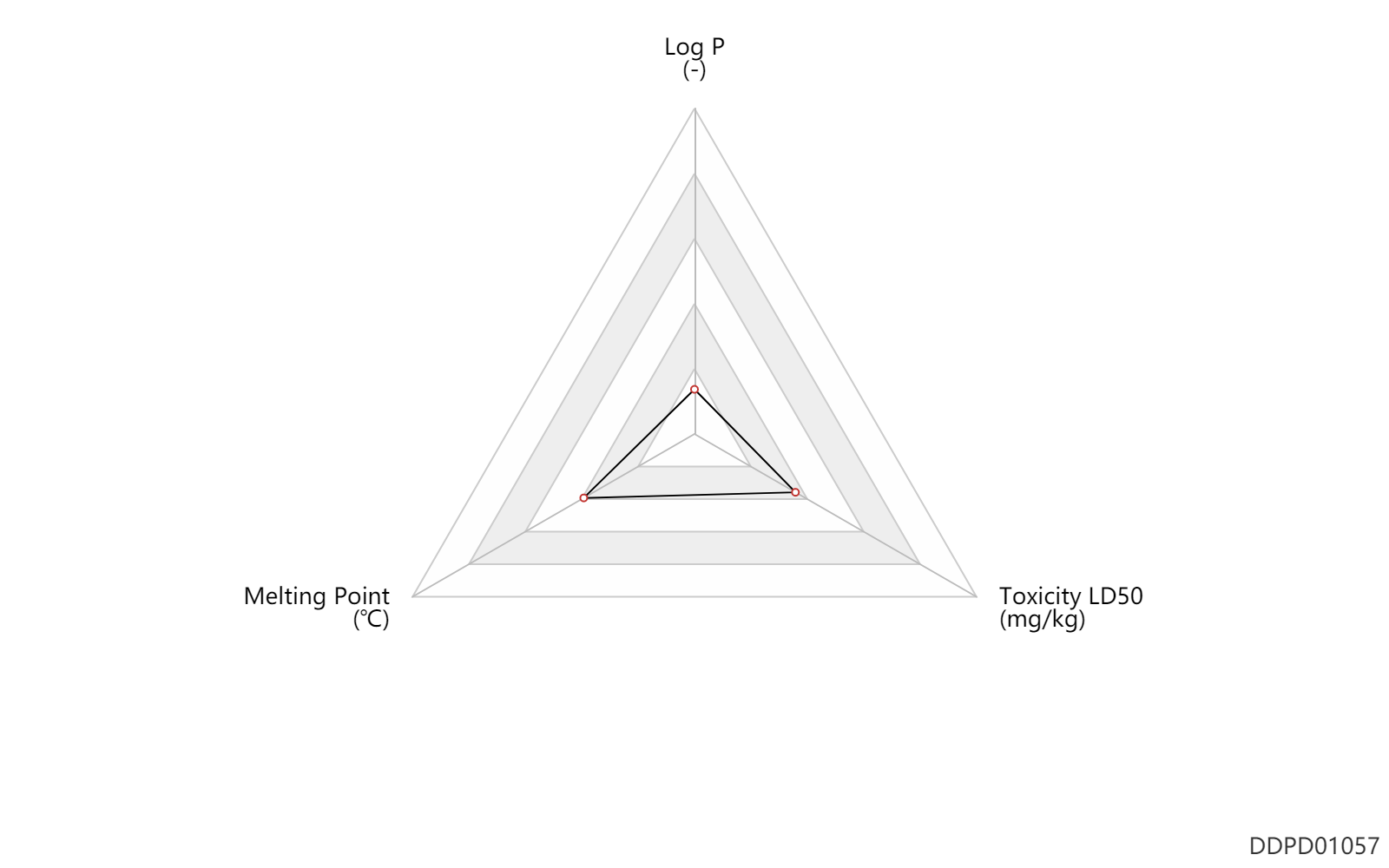
| Log P | -2.25 | - | -2.25 | - | DRUGBANK |
| Melting Point | 124.25 | ℃ | 124-124.5 | ℃ | Fitch, H.M.; U.S. Patent 2,911,430; November 3, 1959; assigned to Campbell Pharmaceuticals, Inc. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Toxicity LD50 | 0.17 | mg/kg | 174.0 | mcg/kg | Rattus, Rat; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | Phospholine Iodide | echothiophate iodide | PDR |
| Max dose for adolescents | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Phospholine Iodide | echothiophate iodide | PDR |
| Max dose for adults | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Phospholine Iodide | echothiophate iodide | PDR |
| Max dose for elderly | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Phospholine Iodide | echothiophate iodide | PDR |