Basic Information
| Drug ID | DDPD01047 |

|
| Drug Name | Fluocinonide | |
| Molecular Weight | 494.5249 | |
| Molecular Formula | C26H32F2O7 | |
| CAS Number | 356-12-7 | |
| SMILES | [H][C@@]12C[C@@]3([H])[C@]4([H])C[C@H](F)C5=CC(=O)C=C[C@]5(C)[C@@]4(F)[C@@H](O)C[C@]3(C)[C@@]1(OC(C)(C)O2)C(=O)COC(C)=O | |
| External Links | ||
| DRUGBANK | DB01047 | |
| PubChem Compound | 9642 | |
| PDR | 325 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
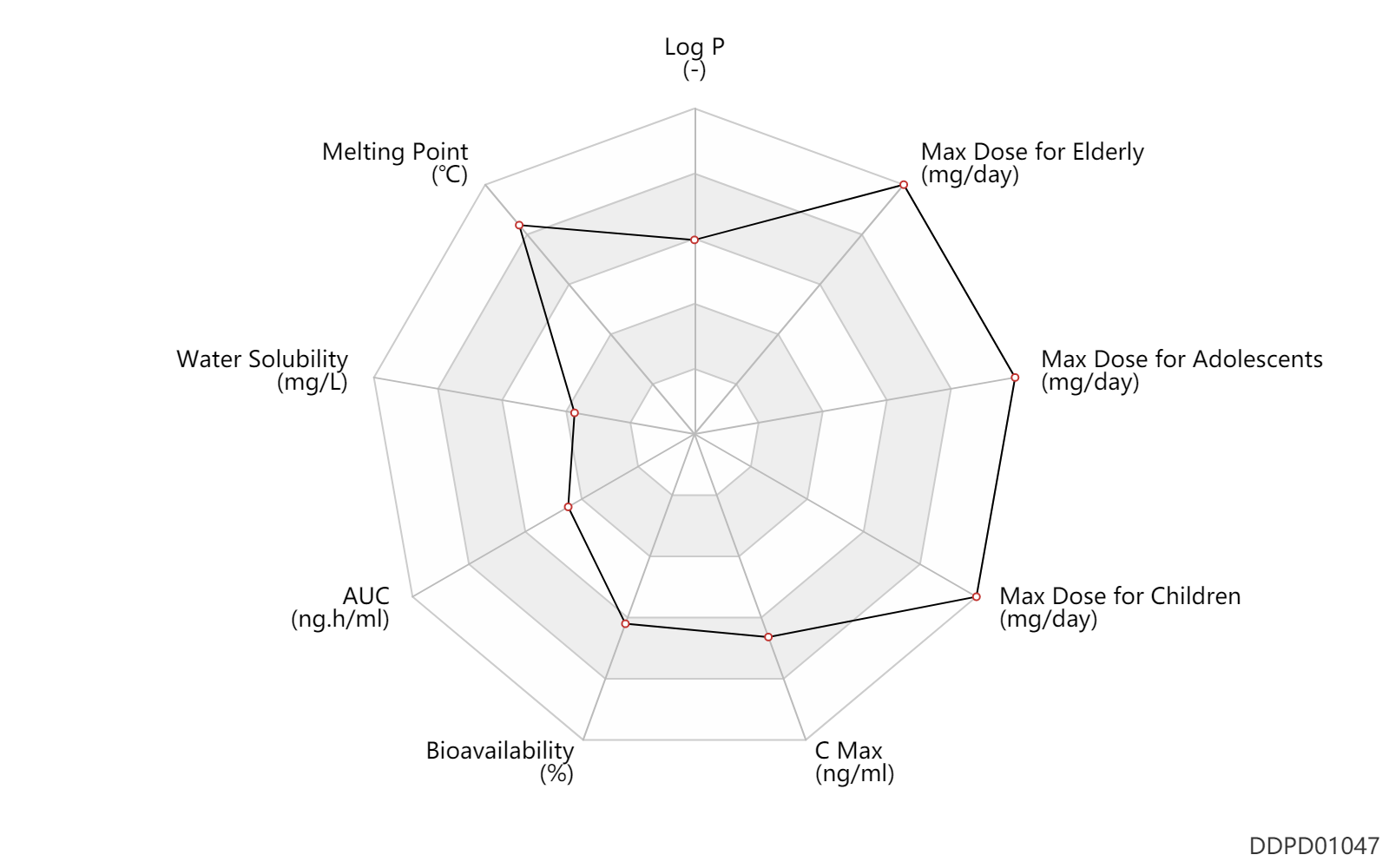
| Log P | 3.19 | - | 3.19 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 309.0 | ℃ | 309 | ℃ | PhysProp |
| Water Solubility | 4.74 | mg/L | 4.74 | mg/L | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 6020.0 | ng.h/ml | 6.02±1.73 | mcg.h/ml | Tablet, PO, oral; | DRUGBANK |
| Bioavailability | 83.0 | % | 83 | % | Tablet, PO, oral; | DRUGBANK |
| C Max | 3000.0 | ng/ml | 3.0±0.89 | mcg/ml | Tablet, PO, oral; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 4.0 | application/day | 4 | application/day | skin/dermal | Vanos | fluocinonide | PDR |
| Max dose for children | 2.0 | application/day | 2 | application/day | skin/dermal | Vanos | fluocinonide | PDR |
| Max dose for children | 8571.42857142 | mg/day | 60 | g/week | up to 2 weeks | Vanos | fluocinonide | PDR |
| Max dose for adolescents | 4.0 | application/day | 4 | application/day | skin/dermal | Vanos | fluocinonide | PDR |
| Max dose for adolescents | 2.0 | application/day | 2 | application/day | skin/dermal | Vanos | fluocinonide | PDR |
| Max dose for adolescents | 8571.42857142857 | mg/day | 60 | g/week | up to 2 weeks | Vanos | fluocinonide | PDR |
| Max dose for elderly | 4.0 | application/day | 4 | application/day | skin/dermal | Vanos | fluocinonide | PDR |
| Max dose for elderly | 2.0 | application/day | 2 | application/day | skin/dermal | Vanos | fluocinonide | PDR |
| Max dose for elderly | 8571.42857142857 | mg/day | 60 | g/week | up to 2 weeks | Vanos | fluocinonide | PDR |