Basic Information
| Drug ID | DDPD01043 |

|
| Drug Name | Memantine | |
| Molecular Weight | 179.3018 | |
| Molecular Formula | C12H21N | |
| CAS Number | 19982-08-2 | |
| SMILES | CC12CC3CC(C)(C1)CC(N)(C3)C2 | |
| External Links | ||
| DRUGBANK | DB01043 | |
| T3DB | T3D2967 | |
| PubChem Compound | 4054 | |
| PDR | 2193 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
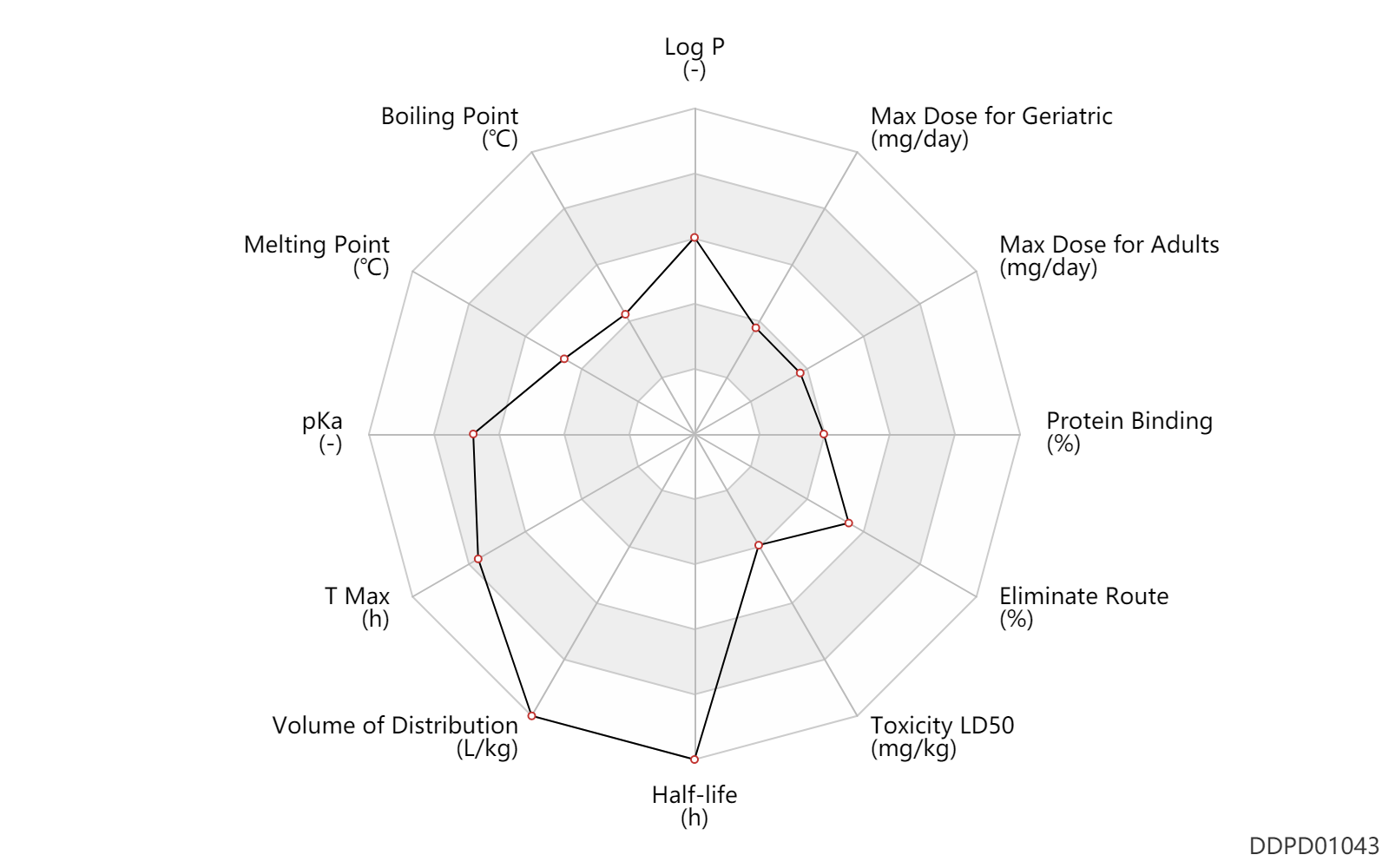
| Log P | 3.28 | - | 3.28 | - | https://www.lundbeck.com/upload/ca/en/files/pdf/pm/Ebixa.pdf |
| Boiling Point | 239.8 | ℃ | 239.8 | ℃ | https://www.lookchem.com/Memantine/ |
| Melting Point | 153.0 | ℃ | 153 | ℃ | https://www.lookchem.com/Memantine/ |
| pKa | 10.27 | - | 10.27 | - | https://www.lundbeck.com/upload/ca/en/files/pdf/pm/Ebixa.pdf |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| T Max | 5.0 | h | 3-7 | h | PO, oral; food; | food → ; | DRUGBANK |
| Volume of Distribution | 10.0 | L/kg | 9.0-11 | L/kg | Average volume of distribution; | DRUGBANK | |
| Half-life | 80.0 | h | 60-100 | h | DRUGBANK | ||
| Toxicity LD50 | 467.5 | mg/kg | 437-498 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 349.0 | mg/kg | 328-370 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 48.0 | % | ~48 | % | Urinary excretion; Unchanged drug; | DRUGBANK | |
| Protein Binding | 45.0 | % | 45 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 20.0 | mg/day | 20 | mg/day | PO, oral | Namenda | memantine hydrochloride | PDR |
| Max dose for adults | 28.0 | mg/day | 28 | mg/day | PO, oral | Namenda | memantine hydrochloride | PDR |
| Max dose for geriatric | 20.0 | mg/day | 20 | mg/day | PO, oral | Namenda | memantine hydrochloride | PDR |
| Max dose for geriatric | 28.0 | mg/day | 28 | mg/day | PO, oral | Namenda | memantine hydrochloride | PDR |