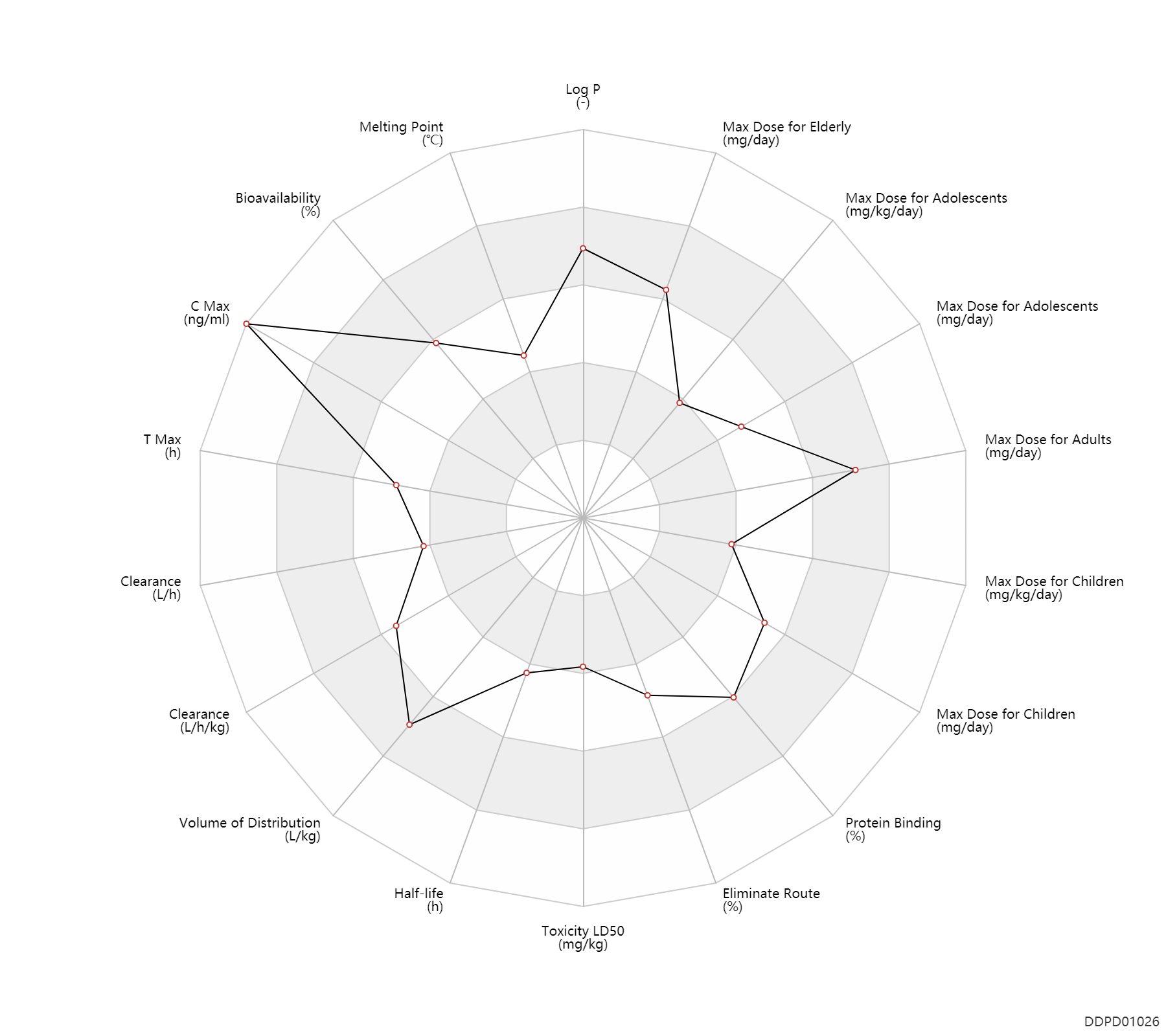
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Bioavailability |
76.0 |
% |
76 |
% |
|
|
DRUGBANK |
| C Max |
3050.0 |
ng/ml |
2.5-3.6 |
mcg/ml |
Oral single dose; |
|
DRUGBANK |
C Max |
1700579.2 |
ng/ml |
3.2(1.4-4.5) |
mM |
PO, oral; superficial mycotic infection; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
2.5 |
h |
1-4 |
h |
Oral single dose; |
|
DRUGBANK |
T Max |
2.0 |
h |
1-3 |
h |
PO, oral; superficial mycotic infection; |
|
The Pharmacological Basis of Therapeutics |
| Clearance |
8.7 |
L/h |
8.66 |
L/h |
|
|
DRUGBANK |
Clearance |
0.50 |
L/h/kg |
8.4±4.1 |
ml/min/kg |
apparent clearance; |
|
The Pharmacological Basis of Therapeutics |
| Volume of Distribution |
0.36 |
L/kg |
0.36 |
L/kg |
|
|
DRUGBANK |
Volume of Distribution |
8.4 |
L/kg |
8.4±4.1 |
L/kg |
Apparent volume of distribution; |
|
The Pharmacological Basis of Therapeutics |
| Half-life |
2.0 |
h |
2 |
h |
elimination half-life; |
|
DRUGBANK |
Half-life |
8.0 |
h |
8 |
h |
terminal half-life; |
|
DRUGBANK |
Half-life |
3.3 |
h |
3.3±1.0 |
h |
|
increasing doses ↑ ; |
The Pharmacological Basis of Therapeutics |
Half-life |
8.0 |
h |
~8 |
h |
Single dose; |
|
The Pharmacological Basis of Therapeutics |
| Toxicity LD50 |
86.0 |
mg/kg |
86.0 |
mg/kg |
PO, oral; Rattus, Rat; |
|
T3DB |
Toxicity LD50 |
44.0 |
mg/kg |
44.0 |
mg/kg |
intravenous injection, IV; mouse; |
|
T3DB |
Toxicity LD50 |
702.0 |
mg/kg |
702.0 |
mg/kg |
PO, oral; mouse; |
|
T3DB |
| Eliminate Route |
95.0 |
% |
>95 |
% |
lung excretion; |
|
DRUGBANK |
Eliminate Route |
3.0 |
% |
2-4 |
% |
Urinary excretion; Unchanged drug; |
|
DRUGBANK |
Eliminate Route |
1.0 |
% |
<1 |
% |
Urinary excretion; Unchanged drug; |
|
The Pharmacological Basis of Therapeutics |
| Protein Binding |
84.0 |
% |
~84 |
% |
plasma proteins; |
|
DRUGBANK |
Protein Binding |
99.0 |
% |
99±0.1 |
% |
|
|
The Pharmacological Basis of Therapeutics |