Basic Information
| Drug ID | DDPD01024 |

|
| Drug Name | Mycophenolic acid | |
| Molecular Weight | 320.3371 | |
| Molecular Formula | C17H20O6 | |
| CAS Number | 24280-93-1 | |
| SMILES | COC1=C(C\C=C(/C)CCC(O)=O)C(O)=C2C(=O)OCC2=C1C | |
| External Links | ||
| DRUGBANK | DB01024 | |
| T3DB | T3D2960 | |
| PubChem Compound | 446541 | |
| PDR | 435 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
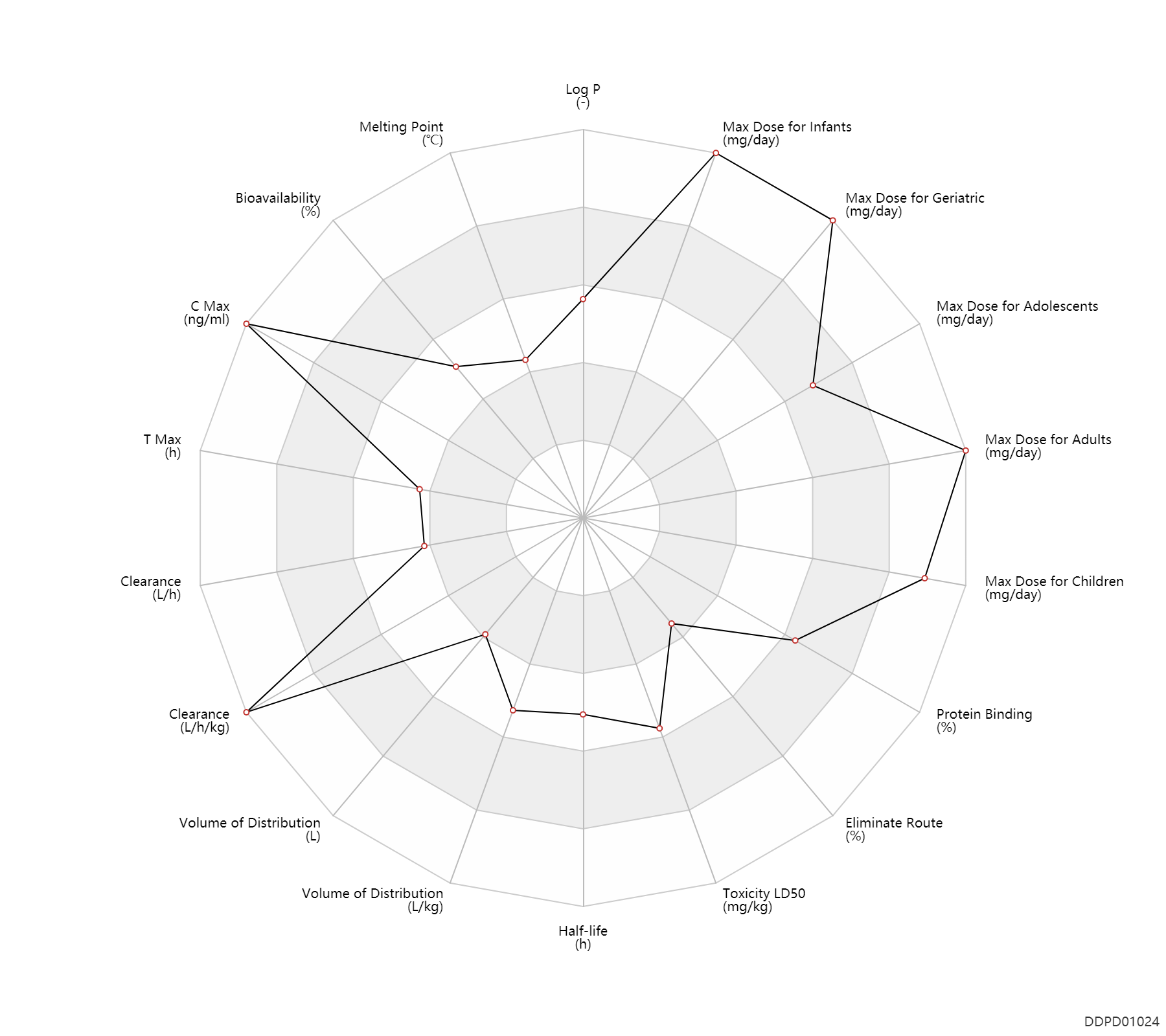
| Log P | 2.8 | - | 2.8 | - | DRUGBANK |
| Melting Point | 141.0 | ℃ | 141 | ℃ | PhysProp |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 82.5 | % | 70-95 | % | Oral single dose; extended release formulation; | DRUGBANK | Bioavailability | 0 | % | ~0 | % | PO, oral; organ transplants; | The Pharmacological Basis of Therapeutics | Bioavailability | 94.0 | % | 94.0 | % | PO, oral; Active metabolite; organ transplants; | The Pharmacological Basis of Therapeutics |
| C Max | 13500.0 | ng/ml | 8-19 | mcg/ml | PO, oral; Active metabolite; organ transplants; | The Pharmacological Basis of Therapeutics | |
| T Max | 1.7 | h | 1.1-2.2 | h | PO, oral; Active metabolite; organ transplants; | The Pharmacological Basis of Therapeutics | |
| Clearance | 8.4 | L/h | 140±30 | ml/min | renal transplant; patients; | DRUGBANK | Clearance | 8.5 | L/h/kg | 120-163 | ml/min/kg | The Pharmacological Basis of Therapeutics | Clearance | 0.15 | L/h/kg | 2.5±0.4 | ml/min/kg | apparent clearance; | hepatopathy,LD → ;RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics | Clearance | 0.14 | L/h/kg | 2.33 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 54.0 | L | 54±25 | L | DRUGBANK | Volume of Distribution | 3.8 | L/kg | 3.6-4 | L/kg | Apparent volume of distribution; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 0.77 | L/kg | 0.77 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 12.0 | h | 8-16 | h | elimination half-life; | DRUGBANK | Half-life | 15.0 | h | 13-17 | h | elimination half-life; | DRUGBANK | Half-life | 0.0330 | h | <0.033 | h | intravenous injection, IV; PO, oral; | The Pharmacological Basis of Therapeutics | Half-life | 16.6 | h | 16.6±5.8 | h | The Pharmacological Basis of Therapeutics | Half-life | 9.7 | h | 9.7 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 352.0 | mg/kg | 352.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 1000.0 | mg/kg | 1000.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 6000.0 | mg/kg | >6000 | mg/kg | PO, oral; rabbit; | DRUGBANK | Toxicity LD50 | 352.0 | mg/kg | 352.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB | Toxicity LD50 | 1000.0 | mg/kg | 1000.0 | mg/kg | PO, oral; mouse; | T3DB | Toxicity LD50 | 6000.0 | mg/kg | >6000 | mg/kg | PO, oral; rabbit; | T3DB |
| Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; Active metabolite; adults; normal,healthy; organ transplants; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics | |
| Protein Binding | 98.0 | % | >98 | % | DRUGBANK | Protein Binding | 97.5 | % | 97.5 | % | Active metabolite; adults; normal,healthy; organ transplants; human, homo sapiens; | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 1200.0 | mg/m2/day | 1200 | mg/m2/day | Liquid | Myfortic | mycophenolic acid | PDR |
| Max dose for infants | 2.0 | mg/day | 2 | mg/day | Liquid | Myfortic | mycophenolic acid | PDR |
| Max dose for infants | 1500.0 | mg/day | 1500 | mg/day | Capsule, PO, Oral | Myfortic | mycophenolic acid | PDR |
| Max dose for infants | 2.0 | g/day | 2 | g/day | Tablet,PO,oral;Capsule, PO, Oral; | Myfortic | mycophenolic acid | PDR |
| Max dose for infants | 800.0 | mg/m2/day | 800 | mg/m2/day | Tablet,PO,oral | Myfortic | mycophenolic acid | PDR |
| Max dose for infants | 1400.0 | mg/day | 1400 | mg/day | Tablet,PO,oral | Myfortic | mycophenolic acid | PDR |
| Max dose for children | 1200.0 | mg/m2/day | 1200 | mg/m2/day | Liquid | Myfortic | mycophenolic acid | PDR |
| Max dose for children | 2.0 | mg/day | 2 | mg/day | Liquid | Myfortic | mycophenolic acid | PDR |
| Max dose for children | 1500.0 | mg/day | 1500 | mg/day | Capsule, PO, Oral | Myfortic | mycophenolic acid | PDR |
| Max dose for children | 2000.0 | mg/day | 2 | g/day | Tablet,PO,oral;Capsule, PO, Oral; | Myfortic | mycophenolic acid | PDR |
| Max dose for children | 800.0 | mg/m2/day | 800 | mg/m2/day | Tablet,PO,oral | Myfortic | mycophenolic acid | PDR |
| Max dose for children | 1400.0 | mg/day | 1400 | mg/day | Tablet,PO,oral | Myfortic | mycophenolic acid | PDR |
| Max dose for adolescents | 1200.0 | mg/m2/day | 1200 | mg/m2/day | Liquid | Myfortic | mycophenolic acid | PDR |
| Max dose for adolescents | 2.0 | mg/day | 2 | mg/day | Liquid | Myfortic | mycophenolic acid | PDR |
| Max dose for adolescents | 1500.0 | mg/day | 1500 | mg/day | Capsule, PO, Oral | Myfortic | mycophenolic acid | PDR |
| Max dose for adolescents | 2000.0 | mg/day | 2 | g/day | Tablet,PO,oral;Capsule, PO, Oral; | Myfortic | mycophenolic acid | PDR |
| Max dose for adolescents | 800.0 | mg/m2/day | 800 | mg/m2/day | Tablet,PO,oral | Myfortic | mycophenolic acid | PDR |
| Max dose for adolescents | 1400.0 | mg/day | 1400 | mg/day | Tablet,PO,oral | Myfortic | mycophenolic acid | PDR |
| Max dose for adults | 3000.0 | mg/day | 3 | g/day | Tablet,PO,oral;Capsule, PO, Oral;intravenous infusion, iv in drop; | Myfortic | mycophenolic acid | PDR |
| Max dose for adults | 1440.0 | mg/day | 1440 | mg/day | Tablet,PO,oral | Myfortic | mycophenolic acid | PDR |
| Max dose for geriatric | 3000.0 | mg/day | 3 | g/day | Tablet,PO,oral;Capsule, PO, Oral;intravenous infusion, iv in drop; | Myfortic | mycophenolic acid | PDR |
| Max dose for geriatric | 1440.0 | mg/day | 1440 | mg/day | Tablet,PO,oral | Myfortic | mycophenolic acid | PDR |