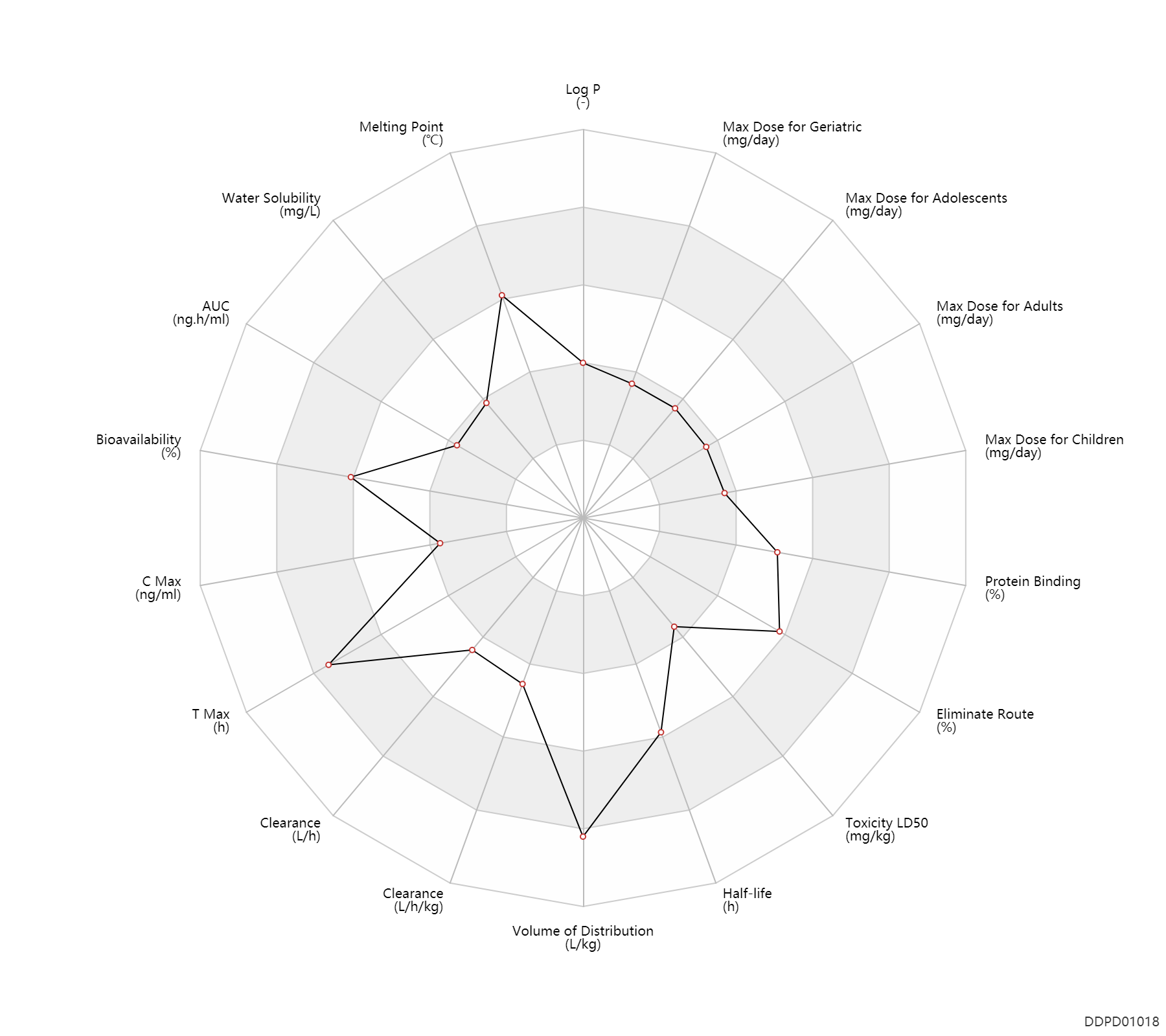
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| AUC |
56.0 |
ng.h/ml |
56±15 |
ng.h/ml |
PO, oral; immediate release formulation; |
|
DRUGBANK |
AUC |
32.0 |
ng.h/ml |
32±9 |
ng.h/ml |
PO, oral; extended release formulation; |
|
DRUGBANK |
| Bioavailability |
80.0 |
% |
80 |
% |
PO, oral; |
|
DRUGBANK |
| C Max |
2.5 |
ng/ml |
2.5±0.6 |
ng/ml |
PO, oral; immediate release formulation; |
|
DRUGBANK |
C Max |
1.0 |
ng/ml |
1.0±0.3 |
ng/ml |
PO, oral; extended release formulation; |
|
DRUGBANK |
C Max |
3.6 |
ng/ml |
3.58±1.39 |
ng/ml |
PO, oral; adults; extended release formulation; |
|
DRUGBANK |
C Max |
2.6 |
ng/ml |
2.6±1.03 |
ng/ml |
PO, oral; Children; extended release formulation; |
|
DRUGBANK |
C Max |
1.7 |
ng/ml |
1.7±0.43 |
ng/ml |
PO, oral; Adolescents; extended release formulation; |
|
DRUGBANK |
| T Max |
3.0 |
h |
3 |
h |
PO, oral; immediate release formulation; |
|
DRUGBANK |
T Max |
6.0 |
h |
6 |
h |
PO, oral; extended release formulation; |
|
DRUGBANK |
T Max |
5.5 |
h |
5.5 |
h |
PO, oral; adults; extended release formulation; |
|
DRUGBANK |
T Max |
5.0 |
h |
4.98 |
h |
PO, oral; Children; extended release formulation; |
|
DRUGBANK |
T Max |
5.0 |
h |
4.96 |
h |
PO, oral; Adolescents; extended release formulation; |
|
DRUGBANK |
| Clearance |
21.6 |
L/h |
360±262 |
ml/min |
Total clearance; normal renal function; patients; |
|
DRUGBANK |
Clearance |
14.0 |
L/h |
233±245 |
ml/min |
Renal clearance; normal renal function; patients; |
|
DRUGBANK |
Clearance |
18.5 |
L/h |
308±274 |
ml/min |
Total clearance; Chronic Kidney Disease; |
|
DRUGBANK |
Clearance |
2.0 |
L/h |
34±22 |
ml/min |
Renal clearance; Chronic Kidney Disease; |
|
DRUGBANK |
Clearance |
15.4 |
L/h |
257±187 |
ml/min |
Total clearance; renal insufficiency; |
|
DRUGBANK |
Clearance |
1.1 |
L/h |
18±15 |
ml/min |
Renal clearance; renal insufficiency; |
|
DRUGBANK |
Clearance |
0.27 |
L/h/kg |
4.5 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
6.3 |
L/kg |
6.3 |
L/kg |
|
|
DRUGBANK |
Volume of Distribution |
5.6 |
L/kg |
5.6 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
17.0 |
h |
17(10-30) |
h |
|
renal insufficiency → ; |
DRUGBANK |
Half-life |
15.0 |
h |
15 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 |
142.0 |
mg/kg |
142.0 |
mg/kg |
PO, oral; Rattus, Rat; |
|
DRUGBANK |
Toxicity LD50 |
15.3 |
mg/kg |
15.3 |
mg/kg |
PO, oral; mouse; |
|
DRUGBANK |
Toxicity LD50 |
114.0 |
mg/kg |
114.0 |
mg/kg |
subcutaneous injection, SC; Rattus, Rat; |
|
DRUGBANK |
Toxicity LD50 |
46.0 |
mg/kg |
46.0 |
mg/kg |
subcutaneous injection, SC; mouse; |
|
DRUGBANK |
| Eliminate Route |
57.0 |
% |
57±32 |
% |
Urinary excretion; normal renal function; patients; |
|
DRUGBANK |
| Protein Binding |
70.0 |
% |
~70 |
% |
|
|
DRUGBANK |