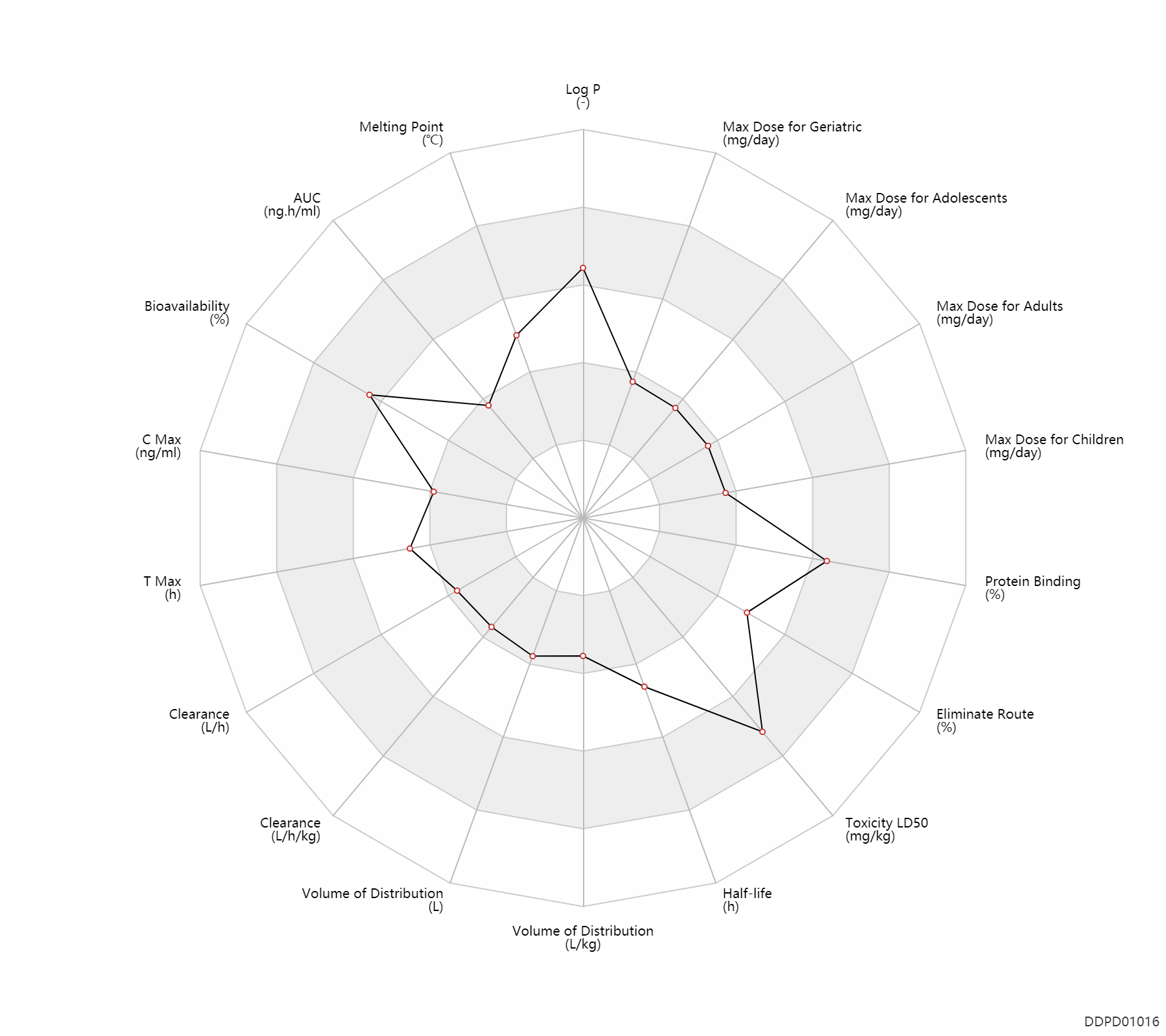
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| AUC |
348.0 |
ng.h/ml |
348.0 |
ng.h/ml |
PO, oral; patients; |
|
DRUGBANK |
| Bioavailability |
95.0 |
% |
90-100 |
% |
Tablet, PO, oral; Drug form; |
|
The Pharmacological Basis of Therapeutics |
Bioavailability |
77.0 |
% |
64-90 |
% |
Tablet, PO, oral; Drug form; |
|
The Pharmacological Basis of Therapeutics |
| C Max |
263.0 |
ng/ml |
211-315 |
ng/ml |
PO, oral; Geriatric; |
|
DRUGBANK |
C Max |
223.0 |
ng/ml |
144-302 |
ng/ml |
PO, oral; adults; patients; |
|
DRUGBANK |
C Max |
106.0 |
ng/ml |
106.0 |
ng/ml |
Tablet, PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
C Max |
104.0 |
ng/ml |
104.0 |
ng/ml |
Tablet, PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
0.95 |
h |
0.9-1 |
h |
PO, oral; Geriatric; |
|
DRUGBANK |
T Max |
2.2 |
h |
1.3-3 |
h |
PO, oral; adults; patients; |
|
DRUGBANK |
T Max |
1.5 |
h |
~1.5 |
h |
Tablet, PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
T Max |
3.0 |
h |
2-4 |
h |
Tablet, PO, oral; Drug form; adults; normal,healthy; |
|
The Pharmacological Basis of Therapeutics |
| Clearance |
3.1 |
L/h |
2.70-3.55 |
L/h |
Geriatric; |
|
DRUGBANK |
Clearance |
3.3 |
L/h |
2.47-4.11 |
L/h |
young; patients; |
|
DRUGBANK |
Clearance |
0.0780 |
L/h/kg |
1.3±0.5 |
ml/min/kg |
|
Hepatic cirrhosis, cirr ↓ ; |
The Pharmacological Basis of Therapeutics |
Clearance |
0.0492 |
L/h/kg |
0.82 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
36.0 |
L |
19.3-52.6 |
L |
Geriatric; |
|
DRUGBANK |
Volume of Distribution |
35.4 |
L |
21.5-49.3 |
L |
young; patients; |
|
DRUGBANK |
Volume of Distribution |
0.20 |
L/kg |
0.20±0.11 |
L/kg |
|
|
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
0.0800 |
L/kg |
0.08 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
8.7 |
h |
4.0-13.4 |
h |
elimination half-life; Geriatric; |
|
DRUGBANK |
Half-life |
9.0 |
h |
4.0-13.9 |
h |
elimination half-life; young; patients; |
|
DRUGBANK |
Half-life |
4.0 |
h |
4±1 |
h |
|
Hepatic cirrhosis, cirr ↑ ;non-insulin-dependent diabetes mellitus NIDDM
↑ ; |
The Pharmacological Basis of Therapeutics |
Half-life |
8.0 |
h |
6-10 |
h |
nonmicronized formulation; |
|
The Pharmacological Basis of Therapeutics |
Half-life |
15.0 |
h |
15 |
h |
terminal half-life; |
|
The Pharmacological Basis of Therapeutics |
Half-life |
2.2 |
h |
2.2 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 |
3200.0 |
mg/kg |
>3200 |
mg/kg |
PO, oral; Rattus, Rat; |
|
DRUGBANK |
Toxicity LD50 |
1500.0 |
mg/kg |
>1500 |
mg/kg |
PO, oral; mouse; |
|
DRUGBANK |
Toxicity LD50 |
10000.0 |
mg/kg |
>10000 |
mg/kg |
PO, oral; rabbit; |
|
DRUGBANK |
Toxicity LD50 |
1500.0 |
mg/kg |
>1500 |
mg/kg |
PO, oral; guinea pigs; |
|
DRUGBANK |
| Eliminate Route |
50.0 |
% |
50 |
% |
Urinary excretion; |
|
DRUGBANK |
Eliminate Route |
50.0 |
% |
50 |
% |
Faeces excretion; |
|
DRUGBANK |
Eliminate Route |
0 |
% |
~0 |
% |
Urinary excretion; Unchanged drug; |
|
The Pharmacological Basis of Therapeutics |
| Protein Binding |
99.9 |
% |
99.9 |
% |
plasma proteins; |
|
DRUGBANK |
Protein Binding |
98.0 |
% |
>98 |
% |
|
|
DRUGBANK |
Protein Binding |
99.8 |
% |
99.8 |
% |
|
Elderly ↓ ; |
The Pharmacological Basis of Therapeutics |