Basic Information
| Drug ID | DDPD01013 |

|
| Drug Name | Clobetasol propionate | |
| Molecular Weight | 466.97 | |
| Molecular Formula | C25H32ClFO5 | |
| CAS Number | 25122-46-7 | |
| SMILES | [H][C@@]12C[C@H](C)[C@](OC(=O)CC)(C(=O)CCl)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C | |
| External Links | ||
| DRUGBANK | DB01013 | |
| PubChem Compound | 32798 | |
| PDR | 2059 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
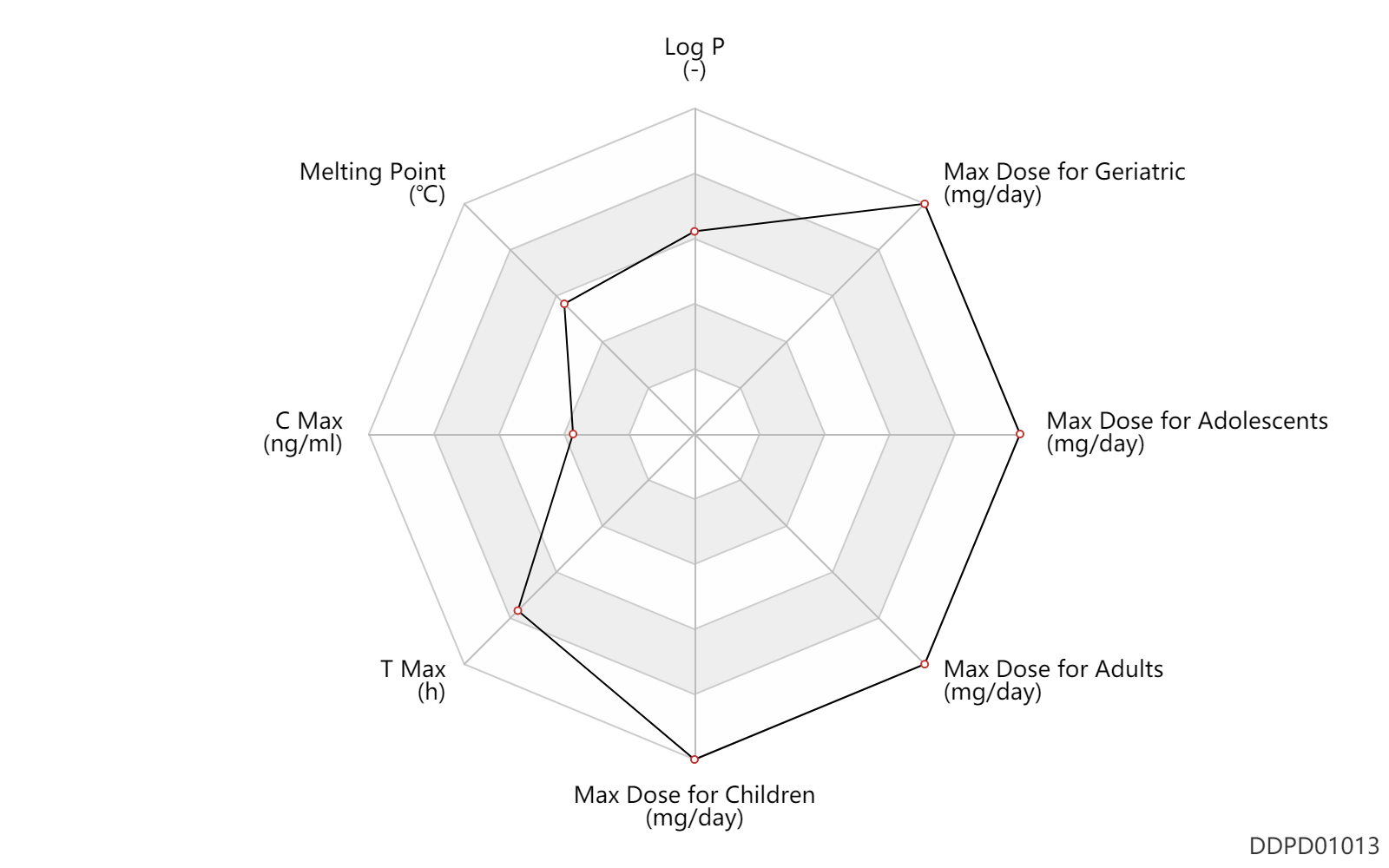
| Log P | 3.5 | - | 3.5 | - | SANGSTER (1994) |
| Melting Point | 196.0 | ℃ | 196 | ℃ | Health Canada Label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| C Max | 0.0590 | ng/ml | 59±36 | pg/ml | skin/dermal; | DRUGBANK | C Max | 0.0563 | ng/ml | 56.3±104.7 | pg/ml | ointment; | DRUGBANK |
| T Max | 5.0 | h | 5 | h | skin/dermal; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 7142.85714285 | mg/day | 50 | g/week | Olux | clobetasol propionate | PDR | |
| Max dose for adolescents | 7142.85714285714 | mg/day | 50 | g/week | Olux | clobetasol propionate | PDR | |
| Max dose for adults | 7.14285714285714 | ml/day | 50 | ml/week | skin/dermal | Olux | clobetasol propionate | PDR |
| Max dose for adults | 8.42857142857143 | ml/day | 59 | ml/week | skin/dermal | Olux | clobetasol propionate | PDR |
| Max dose for adults | 7142.85714285714 | mg/day | 50 | g/week | skin/dermal | Olux | clobetasol propionate | PDR |
| Max dose for geriatric | 50.0 | ml/week | 50 | ml/week | skin/dermal | Olux | clobetasol propionate | PDR |
| Max dose for geriatric | 59.0 | ml/week | 59 | ml/week | skin/dermal | Olux | clobetasol propionate | PDR |
| Max dose for geriatric | 7142.85714285714 | mg/day | 50 | g/week | skin/dermal | Olux | clobetasol propionate | PDR |