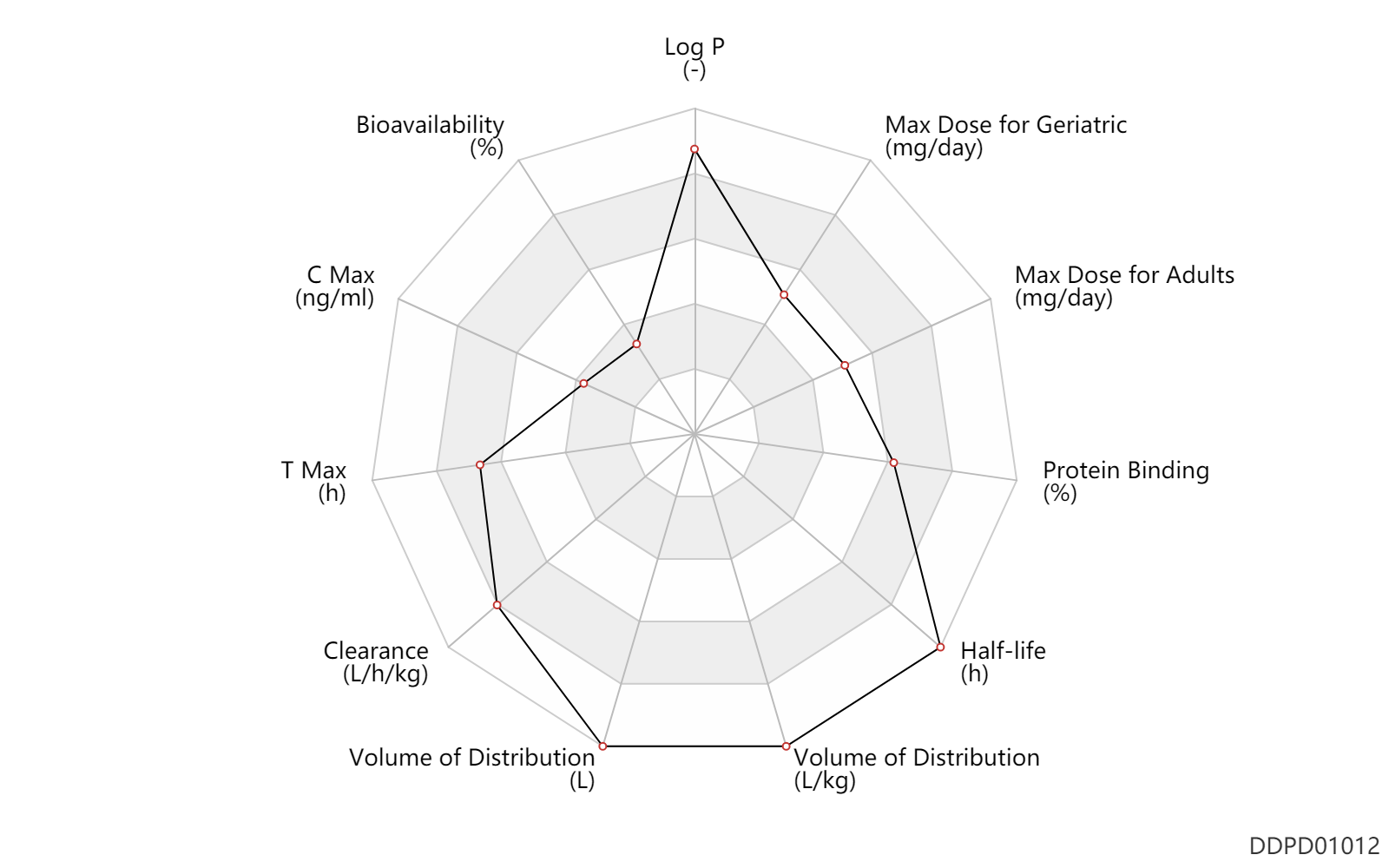
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Bioavailability |
20.0 |
% |
~20 |
% |
PO, oral; |
|
The Pharmacological Basis of Therapeutics |
| C Max |
10.6 |
ng/ml |
10.6±2.8 |
ng/ml |
Oral single dose; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
4.0 |
h |
2-6 |
h |
|
|
The Pharmacological Basis of Therapeutics |
| Clearance |
1.1 |
L/h/kg |
~18 |
ml/min/kg |
hydrolysis; hydrolysis; hydrolysis; |
hepatopathy,LD ↓ ; |
The Pharmacological Basis of Therapeutics |
| Volume of Distribution |
1000.0 |
L |
1000.0 |
L |
|
|
DRUGBANK |
Volume of Distribution |
17.6 |
L/kg |
~17.6 |
L/kg |
hydrolysis; hydrolysis; hydrolysis; |
|
The Pharmacological Basis of Therapeutics |
| Half-life |
35.0 |
h |
30-40 |
h |
terminal half-life; |
|
DRUGBANK |
Half-life |
46.7 |
h |
40-53.3 |
h |
moderate hepatic impairment; |
|
DRUGBANK |
Half-life |
59.5 |
h |
51-68 |
h |
severe hepatic impairment; |
|
DRUGBANK |
Half-life |
34.0 |
h |
34±9 |
h |
|
chronic liver disease ↑ ; |
The Pharmacological Basis of Therapeutics |
| Protein Binding |
95.0 |
% |
93-97 |
% |
plasma proteins; |
|
DRUGBANK |
Protein Binding |
95.0 |
% |
93-97 |
% |
|
|
The Pharmacological Basis of Therapeutics |