Basic Information
| Drug ID | DDPD01008 |

|
| Drug Name | Busulfan | |
| Molecular Weight | 246.302 | |
| Molecular Formula | C6H14O6S2 | |
| CAS Number | 55-98-1 | |
| SMILES | CS(=O)(=O)OCCCCOS(C)(=O)=O | |
| External Links | ||
| DRUGBANK | DB01008 | |
| T3DB | T3D4750 | |
| PubChem Compound | 2478 | |
| PDR | 2213 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
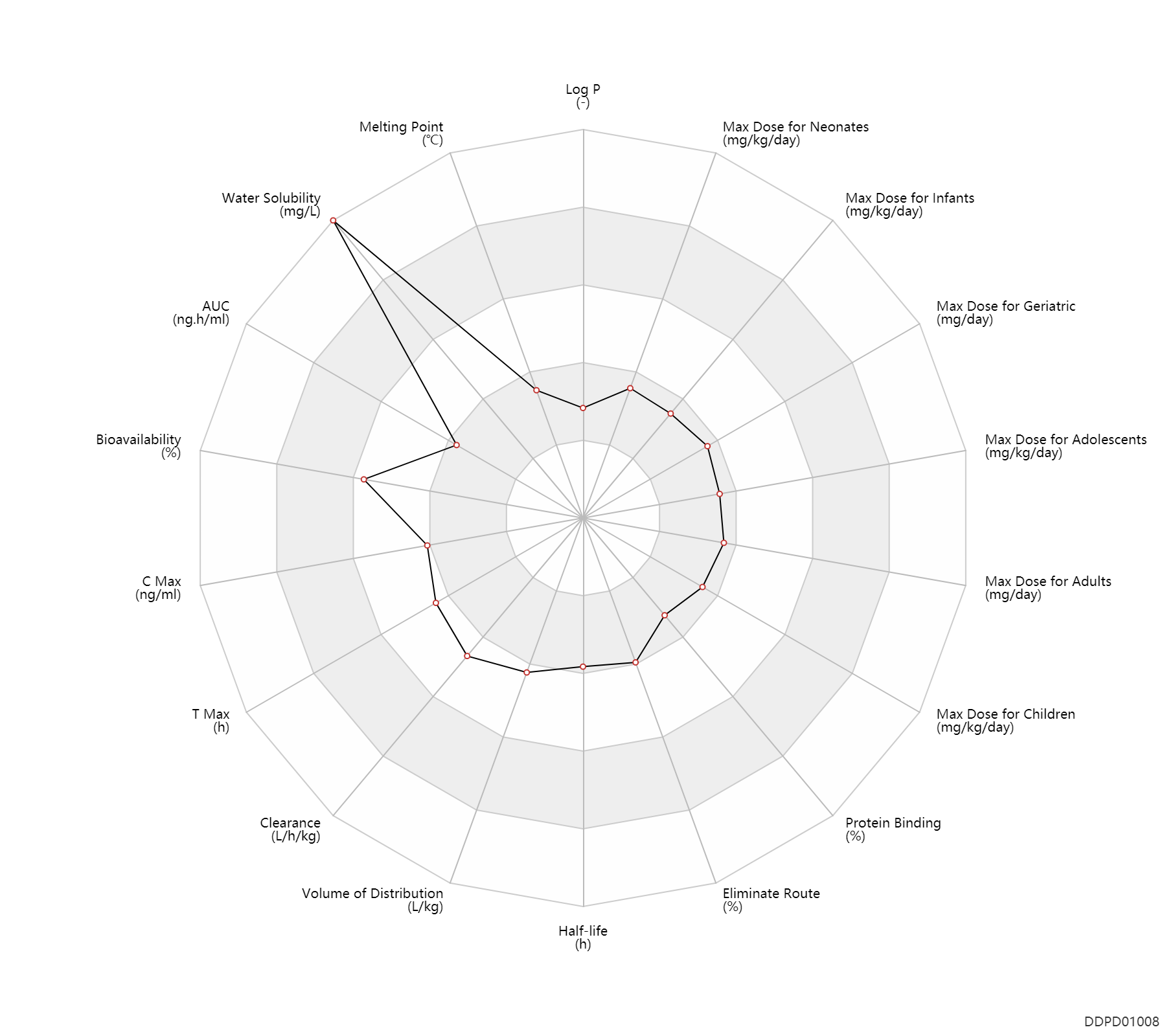
| Log P | -0.52 | - | -0.52 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 106.5 | ℃ | 106-107 | ℃ | Timmis, G.M.; U S . Patent 2,917,432; December 15, 1959; assigned to Burroughs Wellcome & Co., Inc. |
| Water Solubility | 69000.0 | mg/L | 69000 | mg/L | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 130.0 | ng.h/ml | 130.0 | ng.h/ml | Oral single dose; patients; | DRUGBANK |
| Bioavailability | 80.0 | % | 80±20 | % | adults; | DRUGBANK | Bioavailability | 68.0 | % | 68±31 | % | Children; | DRUGBANK | Bioavailability | 70.0 | % | 70(44-94) | % | PO, oral; | The Pharmacological Basis of Therapeutics |
| C Max | 30.0 | ng/ml | 30 | ng/ml | Oral single dose; patients; | DRUGBANK | C Max | 65.0 | ng/ml | 65±27 | ng/ml | Oral single dose; Chronic myelogenous leukemia; | The Pharmacological Basis of Therapeutics | C Max | 949.0 | ng/ml | 949±278 | ng/ml | Oral single dose; bone marrow transplant; | The Pharmacological Basis of Therapeutics |
| T Max | 0.90 | h | 0.9 | h | Oral single dose; patients; | DRUGBANK | T Max | 2.6 | h | 2.6±1.5 | h | PO, oral; | The Pharmacological Basis of Therapeutics |
| Clearance | 0.31 | L/h/kg | 5.2 | ml/min/kg | intravenous infusion, IV in drop; | DRUGBANK | Clearance | 0.27 | L/h/kg | 4.5±0.9 | ml/min/kg | apparent clearance; | The Pharmacological Basis of Therapeutics |
| Volume of Distribution | 0.99 | L/kg | 0.99±0.23 | L/kg | Steady state volume of distribution; | The Pharmacological Basis of Therapeutics |
| Half-life | 2.6 | h | 2.6 | h | DRUGBANK | Half-life | 2.6 | h | 2.6±0.5 | h | The Pharmacological Basis of Therapeutics |
| Eliminate Route | 30.0 | % | ~30 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 2.0 | % | <2 | % | Urinary excretion; Unchanged drug; | DRUGBANK | Eliminate Route | 1.0 | % | 1 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 32.0 | % | 32 | % | plasma proteins; | DRUGBANK | Protein Binding | 47.0 | % | 47 | % | serum albumin; | DRUGBANK | Protein Binding | 8.4 | % | 2.7-14 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 0.06 | mg/kg/day | 60 | mcg/kg/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for neonates | 1.8 | mg/m2/day | 1.8 | mg/m2/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for infants | 0.06 | mg/kg/day | 60 | mcg/kg/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for infants | 1.8 | mg/m2/day | 1.8 | mg/m2/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for children | 0.06 | mg/kg/day | 60 | mcg/kg/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for children | 1.8 | mg/m2/day | 1.8 | mg/m2/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for adolescents | 0.06 | mg/kg/day | 60 | mcg/kg/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for adolescents | 1.8 | mg/m2/day | 1.8 | mg/m2/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for adults | 8.0 | mg/day | 8 | mg/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for adults | 1.0 | mg/kg | 1 | mg/kg | PO, oral | Myleran | busulfan | PDR |
| Max dose for adults | 0.8 | mg/kg | 0.8 | mg/kg | intravenous injection, IV | Myleran | busulfan | PDR |
| Max dose for adults | 1.0 | mg/kg | 1 | mg/kg | intravenous injection, IV | Myleran | busulfan | PDR |
| Max dose for geriatric | 8.0 | mg/day | 8 | mg/day | PO, oral | Myleran | busulfan | PDR |
| Max dose for geriatric | 1.0 | mg/kg | 1 | mg/kg | PO, oral | Myleran | busulfan | PDR |
| Max dose for geriatric | 0.8 | mg/kg | 0.8 | mg/kg | intravenous injection, IV | Myleran | busulfan | PDR |
| Max dose for geriatric | 1.0 | mg/kg | 1 | mg/kg | intravenous injection, IV | Myleran | busulfan | PDR |