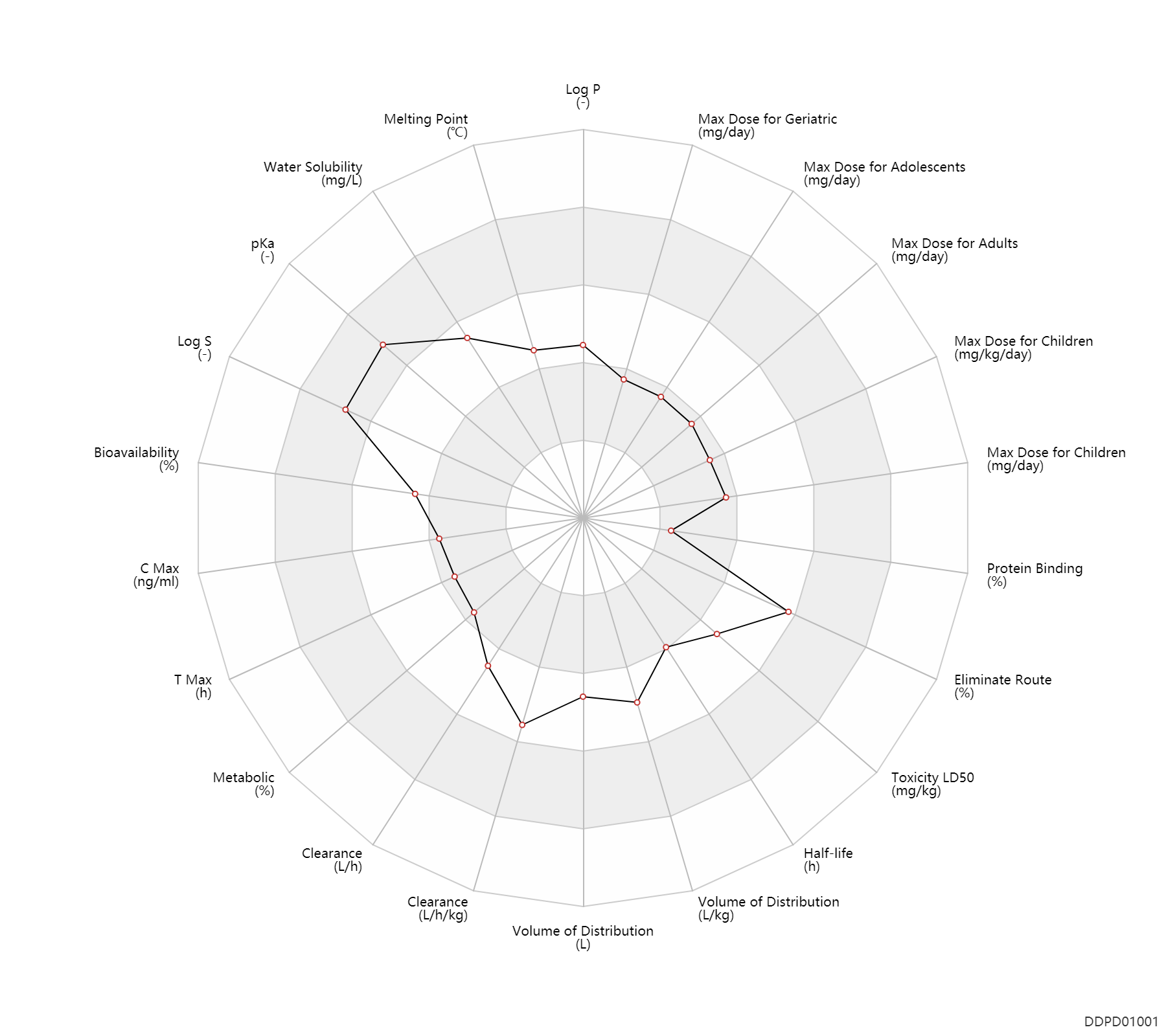
Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | 1.4 | - | 1.4 | - | DRUGBANK |
| Melting Point | 148.0 | ℃ | 147-149 | ℃ | Lunts, L.H.C. and Toon, P.; U.S. Patent 3,644,353; February 22,1972; assigned to Allen & Hanburys Ltd. |
| Water Solubility | 14100.0 | mg/L | 14100 | mg/L | YALKOWSKY,SH & HE,Y 2003) |
| pKa | 10.3 | - | 10.3 | - | DRUGBANK |
| Log S | -1.22 | - | -1.22 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 30.0 | % | 30±7 | % | PO, oral; Optical rotation R; | The Pharmacological Basis of Therapeutics | Bioavailability | 71.0 | % | 71±9 | % | PO, oral; Optical rotation S; | The Pharmacological Basis of Therapeutics | Bioavailability | 25.0 | % | 25.0 | % | inhalation, IH; Optical rotation R; | The Pharmacological Basis of Therapeutics | Bioavailability | 47.0 | % | 47.0 | % | inhalation, IH; Optical rotation S; | The Pharmacological Basis of Therapeutics |
| C Max | 3.0 | ng/ml | 3 | ng/ml | PO, oral; Active metabolite; | DRUGBANK | C Max | 3.6 | ng/ml | 3.6(1.9-5.9) | ng/ml | Oral single dose; Optical rotation R; | The Pharmacological Basis of Therapeutics | C Max | 11.4 | ng/ml | 11.4(7.1-16.2) | ng/ml | Oral single dose; Optical rotation S; | The Pharmacological Basis of Therapeutics |
| T Max | 0.17 | h | 0.17 | h | PO, oral; Active metabolite; | DRUGBANK | T Max | 0.42 | h | 0.42 | h | PO, oral; Active metabolite; | DRUGBANK | T Max | 1.5 | h | 1.5 | h | Oral single dose; Optical rotation R; | The Pharmacological Basis of Therapeutics | T Max | 2.0 | h | 2.0 | h | Oral single dose; Optical rotation S; | The Pharmacological Basis of Therapeutics |
| Metabolic | 0 | % | 0 | % | lung metabolism; | DRUGBANK | |
| Clearance | 16.3 | L/h | 272±38 | ml/min | Renal clearance; PO, oral; | DRUGBANK | Clearance | 17.5 | L/h | 291±70 | ml/min | Renal clearance; intravenous injection, IV; | DRUGBANK | Clearance | 5.9 | L/h | 98.5±23.5 | ml/min | Renal clearance; PO, oral; | DRUGBANK | Clearance | 0.62 | L/h/kg | 10.3±3.0 | ml/min/kg | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics | Clearance | 0.39 | L/h/kg | 6.5±2.0 | ml/min/kg | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics |
| Volume of Distribution | 156.0 | L | 156±38 | L | intravenous injection, IV; | DRUGBANK | Volume of Distribution | 2.0 | L/kg | 2.00±0.70 | L/kg | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 1.8 | L/kg | 1.77±0.69 | L/kg | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics |
| Half-life | 3.9 | h | 2.7-5 | h | elimination half-life; PO, oral; | DRUGBANK | Half-life | 3.9 | h | 2.7-5 | h | elimination half-life; inhalation, IH; | DRUGBANK | Half-life | 4.6 | h | ~4.6 | h | terminal half-life; | DRUGBANK | Half-life | 2.0 | h | 2.00±0.49 | h | normal,healthy; Optical rotation R; | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics | Half-life | 2.9 | h | 2.85±0.85 | h | normal,healthy; Optical rotation S; | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics |
| Toxicity LD50 | 1100.0 | mg/kg | 1100.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 1100.0 | mg/kg | 1100.0 | mg/kg | PO, oral; mouse; | T3DB |
| Eliminate Route | 68.0 | % | 58-78 | % | Urinary excretion; PO, oral; | DRUGBANK | Eliminate Route | 46.0 | % | 46±8 | % | Urinary excretion; Optical rotation R; normal,healthy; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics | Eliminate Route | 55.0 | % | 55±11 | % | Urinary excretion; Optical rotation S; normal,healthy; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 7.0 | % | 7±1 | % | Optical rotation R/S; normal,healthy; human, homo sapiens; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 24.0 | mg/day | 24 | mg/day | Tablet,PO,oral;Liquid; | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 12.0 | puffs/day | 12 | puffs/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 4.0 | dose/day | 4 | dose/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 10.0 | mg/day | 10 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 2.5 | mg/day | 2.5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 5.0 | mg/day | 5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 0.6 | mg/kg/day | 0.6 | mg/kg/day | Liquid | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 12.0 | mg/day | 12 | mg/day | Liquid | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 12.0 | puffs/day | 12 | puffs/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 4.0 | dose/day | 4 | dose/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 10.0 | mg/day | 10 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 2.5 | mg/day | 2.5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 5.0 | mg/day | 5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 0.6 | mg/kg/day | 0.6 | mg/kg/day | Liquid | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 12.0 | mg/day | 12 | mg/day | Liquid | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 4.0 | dose/day | 4 | dose/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 10.0 | mg/day | 10 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 2.5 | mg/day | 2.5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for children | 5.0 | mg/day | 5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 32.0 | mg/day | 32 | mg/day | Tablet,PO,oral;Liquid; | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 12.0 | puffs/day | 12 | puffs/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 4.0 | dose/day | 4 | dose/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 10.0 | mg/day | 10 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 2.5 | mg/day | 2.5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 5.0 | mg/day | 5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 24.0 | mg/day | 24 | mg/day | Liquid | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 32.0 | mg/day | 32 | mg/day | Tablet,PO,oral | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 12.0 | puffs/day | 12 | puffs/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 4.0 | dose/day | 4 | dose/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 10.0 | mg/day | 10 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 2.5 | mg/day | 2.5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adolescents | 5.0 | mg/day | 5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adults | 32.0 | mg/day | 32 | mg/day | Tablet,PO,oral;Liquid; | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adults | 12.0 | puffs/day | 12 | puffs/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adults | 4.0 | dose/day | 4 | dose/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adults | 10.0 | mg/day | 10 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adults | 2.5 | mg/day | 2.5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for adults | 5.0 | mg/day | 5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for geriatric | 32.0 | mg/day | 32 | mg/day | Tablet,PO,oral;Liquid; | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for geriatric | 12.0 | puffs/day | 12 | puffs/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for geriatric | 4.0 | dose/day | 4 | dose/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for geriatric | 10.0 | mg/day | 10 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for geriatric | 2.5 | mg/day | 2.5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |
| Max dose for geriatric | 5.0 | mg/day | 5 | mg/day | inhalation, IH | Albuterol Sulfate Inhalation Solution 0.5% | albuterol sulfate | PDR |