Basic Information
| Drug ID | DDPD00999 |

|
| Drug Name | Hydrochlorothiazide | |
| Molecular Weight | 297.739 | |
| Molecular Formula | C7H8ClN3O4S2 | |
| CAS Number | 58-93-5 | |
| SMILES | NS(=O)(=O)C1=C(Cl)C=C2NCNS(=O)(=O)C2=C1 | |
| External Links | ||
| DRUGBANK | DB00999 | |
| T3DB | T3D4781 | |
| PubChem Compound | 3639 | |
| PDR | 1973 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
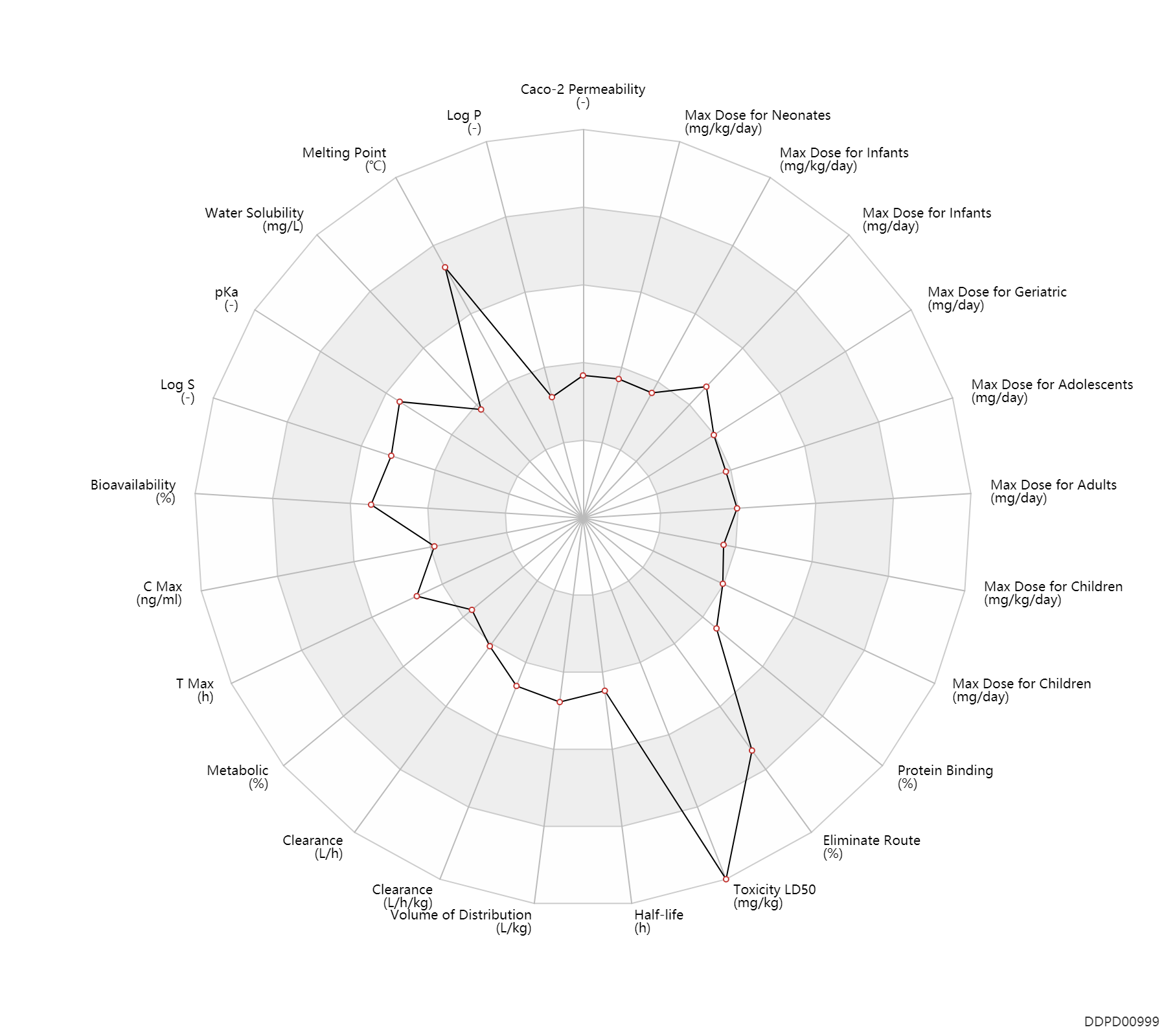
| Caco-2 Permeability | -6.06 | - | -6.06 | - | ADME Research, USCD |
| Log P | -0.07 | - | -0.07 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 267.0 | ℃ | 266-268 | ℃ | U.S. Patent 3,163,645. |
| Water Solubility | 722.0 | mg/L | 722 | mg/L | YALKOWSKY,SH & DANNENFELSER,RM 1992) |
| pKa | 7.9 | - | 7.9 | - | SANGSTER (1994) |
| Log S | -2.62 | - | -2.62 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 70.0 | % | 65-75 | % | PO, oral; | DRUGBANK | Bioavailability | 60.0 | % | 55-65 | % | PO, oral; food; | DRUGBANK | Bioavailability | 71.0 | % | 71±15 | % | PO, oral; | The Pharmacological Basis of Therapeutics |
| C Max | 280.0 | ng/ml | 70-490 | ng/ml | PO, oral; | DRUGBANK | C Max | 224.0 | ng/ml | 56-392 | ng/ml | PO, oral; food; | DRUGBANK | C Max | 75.0 | ng/ml | 75±17 | ng/ml | Oral single dose; adults; normal,healthy; | The Pharmacological Basis of Therapeutics | C Max | 91.0 | ng/ml | 91±0.2 | ng/ml | Oral multiple dose; adults; normal,healthy; | The Pharmacological Basis of Therapeutics |
| T Max | 1.6 | h | 1.6(1-5) | h | PO, oral; | DRUGBANK | T Max | 2.9 | h | 2.9 | h | PO, oral; food; | DRUGBANK | T Max | 1.9 | h | 1.9±0.5 | h | Oral single dose; adults; normal,healthy; | The Pharmacological Basis of Therapeutics | T Max | 2.0 | h | 2.0 | h | Oral multiple dose; adults; normal,healthy; | The Pharmacological Basis of Therapeutics |
| Metabolic | 0 | % | 0 | % | DRUGBANK | ||
| Clearance | 17.1 | L/h | 285.0 | ml/min | Renal clearance; normal renal function; patients; | DRUGBANK | Clearance | 4.5 | L/h | 75.0 | ml/min | Renal clearance; mild renal function; moderate renal function; | DRUGBANK | Clearance | 1.0 | L/h | 17.0 | ml/min | Renal clearance; severe renal function; | DRUGBANK | Clearance | 0.29 | L/h/kg | 4.9±1.1 | ml/min/kg | Elderly ↓ ;RD, renal impairment, Renal disease,including uremia ↓ ;congestive heart disease ↓ ; | The Pharmacological Basis of Therapeutics |
| Volume of Distribution | 2.5 | L/kg | 0.83-4.19 | L/kg | DRUGBANK | Volume of Distribution | 0.83 | L/kg | 0.83±0.31 | L/kg | Elderly ↓ ; | The Pharmacological Basis of Therapeutics |
| Half-life | 10.2 | h | 5.6-14.8 | h | elimination half-life; | DRUGBANK | Half-life | 2.5 | h | 2.5±0.2 | h | The Pharmacological Basis of Therapeutics | Half-life | 8.0 | h | 8±2.8 | h | terminal half-life; | RD, renal impairment, Renal disease,including uremia ↑ ;congestive heart disease ↑ ;Age ↑ ; | The Pharmacological Basis of Therapeutics |
| Toxicity LD50 | 10000.0 | mg/kg | >10 | g/kg | PO, oral; mouse; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 10000.0 | mg/kg | >10 | g/kg | PO, oral; mouse; Rattus, Rat; | T3DB |
| Eliminate Route | 95.0 | % | >95 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics | |
| Protein Binding | 54.0 | % | 40-68 | % | plasma proteins; | DRUGBANK | Protein Binding | 58.0 | % | 58±17 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 3.0 | mg/kg/day | 3 | mg/kg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for neonates | 3.3 | mg/kg/day | 3.3 | mg/kg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for infants | 2.0 | mg/kg/day | 2 | mg/kg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for infants | 3.0 | mg/kg/day | 3 | mg/kg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for infants | 3.3 | mg/kg/day | 3.3 | mg/kg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for infants | 37.5 | mg/day | 37.5 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for children | 2.0 | mg/kg/day | 2 | mg/kg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for children | 2.0 | mg/kg/day | 2 | mg/kg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for children | 37.5 | mg/day | 37.5 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for children | 100.0 | mg/day | 100 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for adolescents | 50.0 | mg/day | 50 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for adolescents | 100.0 | mg/day | 100 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for adults | 50.0 | mg/day | 50 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for adults | 100.0 | mg/day | 100 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for geriatric | 50.0 | mg/day | 50 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |
| Max dose for geriatric | 100.0 | mg/day | 100 | mg/day | PO, oral | Hydrochlorothiazide Tablets | hydrochlorothiazide | PDR |