Basic Information
| Drug ID | DDPD00997 |

|
| Drug Name | Doxorubicin | |
| Molecular Weight | 543.5193 | |
| Molecular Formula | C27H29NO11 | |
| CAS Number | 23214-92-8 | |
| SMILES | COC1=CC=CC2=C1C(=O)C1=C(O)C3=C(C[C@](O)(C[C@@H]3O[C@H]3C[C@H](N)[C@H](O)[C@H](C)O3)C(=O)CO)C(O)=C1C2=O | |
| External Links | ||
| DRUGBANK | DB00997 | |
| T3DB | T3D2953 | |
| PubChem Compound | 31703 | |
| PDR | 1379 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
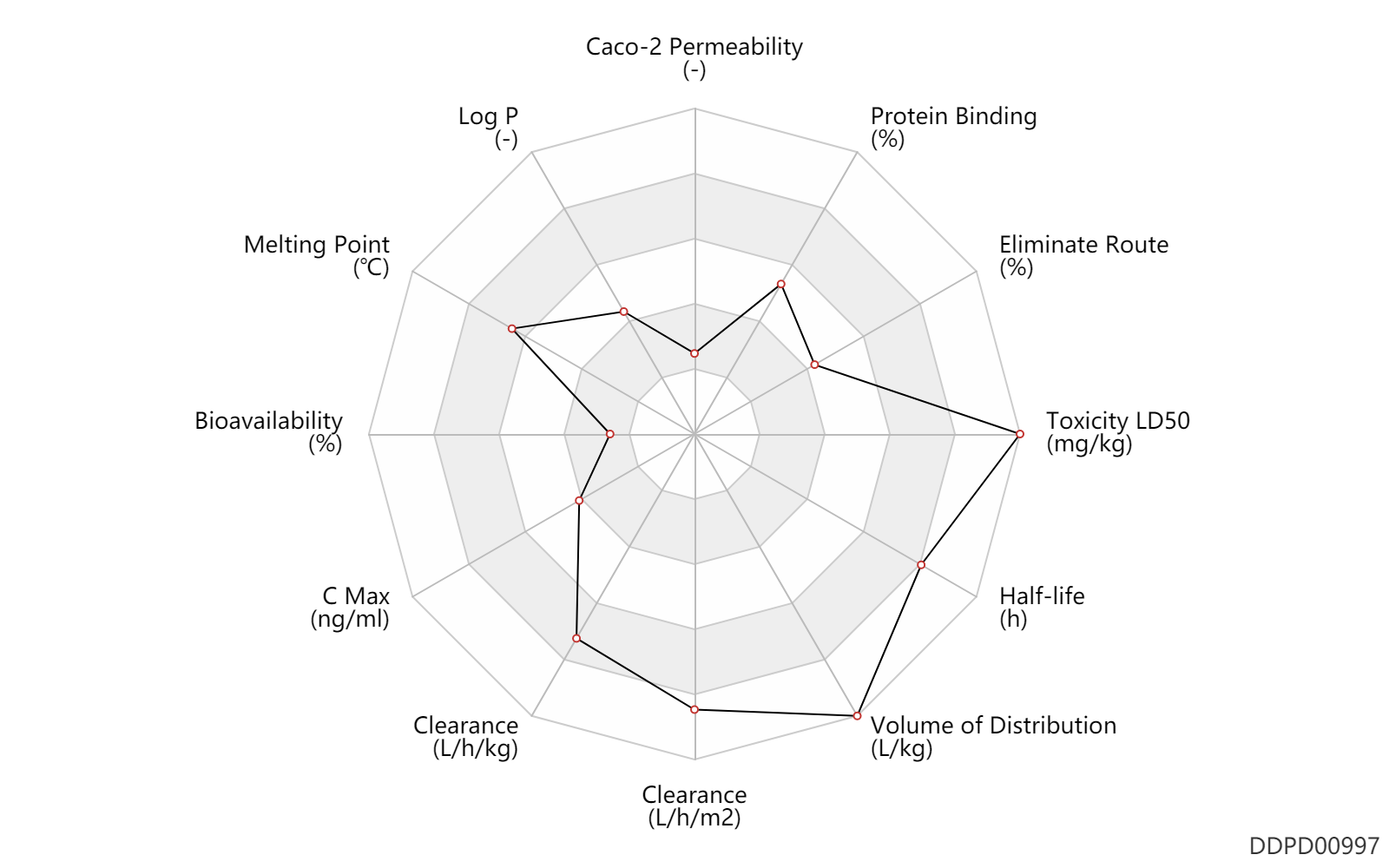
| Caco-2 Permeability | -6.8 | - | -6.8 | - | ADME Research, USCD |
| Log P | 1.27 | - | 1.27 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 230.0 | ℃ | 229-231 | ℃ | PhysProp |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 5.0 | % | 5.0 | % | PO, oral; | The Pharmacological Basis of Therapeutics | |
| C Max | 950.0 | ng/ml | ~950 | ng/ml | intravenous infusion, IV in drop; depression; | The Pharmacological Basis of Therapeutics | C Max | 519.0 | ng/ml | 30-1008 | ng/ml | intravenous infusion, IV in drop; Active metabolite; depression; | The Pharmacological Basis of Therapeutics | C Max | 6.0 | ng/ml | 6.0±3.2 | ng/ml | intravenous infusion, IV in drop; Advanced tumors; | The Pharmacological Basis of Therapeutics | C Max | 5.0 | ng/ml | 5.0±3.5 | ng/ml | intravenous infusion, IV in drop; Active metabolite; Advanced tumors; | The Pharmacological Basis of Therapeutics |
| Clearance | 34.0 | L/h/m2 | 324-809 | ml/min/m2 | Bile metabolism; | DRUGBANK | Clearance | 65.3 | L/h/m2 | 1088.0 | ml/min/m2 | Male, men; | DRUGBANK | Clearance | 26.0 | L/h/m2 | 433.0 | ml/min/m2 | Female, women; | DRUGBANK | Clearance | 92.4 | L/h/m2 | 1540.0 | ml/min/m2 | Children; | DRUGBANK | Clearance | 48.8 | L/h/m2 | 813.0 | ml/min/m2 | Infants; | DRUGBANK | Clearance | 40.0 | L/h/m2 | 666±339 | ml/min/m2 | Children ↑ ;Hepatic cirrhosis, cirr ↓ ;Obesity ↓ ; | The Pharmacological Basis of Therapeutics | Clearance | 0.90 | L/h/kg | 15 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 1011.5 | L/m2 | 809-1214 | L/m2 | Steady state volume of distribution; | DRUGBANK | Volume of Distribution | 682.0 | L/m2 | 682±433 | L/m2 | Hepatic cirrhosis, cirr → ; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 22.0 | L/kg | 22 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 34.0 | h | 20-48 | h | DRUGBANK | Half-life | 26.0 | h | 26±17 | h | RD, renal impairment, Renal disease,including uremia → ;Hepatic cirrhosis, cirr ↑ ; | The Pharmacological Basis of Therapeutics | Half-life | 29.0 | h | 29±16 | h | Active metabolite; | The Pharmacological Basis of Therapeutics | Half-life | 32.0 | h | 32 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 21800.0 | mg/kg | 21800.0 | mg/kg | subcutaneous injection, SC; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 21.8 | mg/kg | 21 800 | ug/kg | subcutaneous injection, SC; Rattus, Rat; | T3DB |
| Eliminate Route | 40.0 | % | 40 | % | Bile excretion; | DRUGBANK | Eliminate Route | 8.5 | % | 5-12 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 7.0 | % | <7 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 75.0 | % | 74-76 | % | plasma proteins; | DRUGBANK | Protein Binding | 76.0 | % | 76 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for children | 550.0 | mg/m2/lifetime | 550 | mg/m2/lifetime | intravenous injection, IV | Adriamycin | doxorubicin hydrochloride | PDR | |
| Max dose for children | 450.0 | mg/m2/lifetime | 450 | mg/m2/lifetime | intravenous injection, IV | Adriamycin | doxorubicin hydrochloride | PDR | |
| Max dose for adolescents | 550.0 | mg/m2/lifetime | 550 | mg/m2/lifetime | intravenous injection, IV | Adriamycin | doxorubicin hydrochloride | PDR | |
| Max dose for adolescents | 450.0 | mg/m2/lifetime | 450 | mg/m2/lifetime | intravenous injection, IV | Adriamycin | doxorubicin hydrochloride | PDR | |
| Max dose for geriatric | 550.0 | mg/m2/lifetime | 550 | mg/m2/lifetime | intravenous injection, IV | Adriamycin | doxorubicin hydrochloride | PDR | |
| Max dose for geriatric | 450.0 | mg/m2/lifetime | 450 | mg/m2/lifetime | intravenous injection, IV | Adriamycin | doxorubicin hydrochloride | PDR | |
| Max dose for adults | 50.0 | mg/m2/dose | 50 | mg/m2/dose | intravenous injection, IV | q4w | Doxorubicin Hydrochloride Liposome | doxorubicin hydrochloride liposome | PDR |
| Max dose for adults | 20.0 | mg/m2/dose | 20 | mg/m2/dose | intravenous injection, IV | q3w | Doxorubicin Hydrochloride Liposome | doxorubicin hydrochloride liposome | PDR |
| Max dose for adults | 30.0 | mg/m2/dose | 30 | mg/m2/dose | intravenous injection, IV | q3w | Doxorubicin Hydrochloride Liposome | doxorubicin hydrochloride liposome | PDR |
| Max dose for geriatric | 50.0 | mg/m2/dose | 50 | mg/m2/dose | intravenous injection, IV | q4w | Doxorubicin Hydrochloride Liposome | doxorubicin hydrochloride liposome | PDR |
| Max dose for geriatric | 20.0 | mg/m2/dose | 20 | mg/m2/dose | intravenous injection, IV | q3w | Doxorubicin Hydrochloride Liposome | doxorubicin hydrochloride liposome | PDR |
| Max dose for geriatric | 30.0 | mg/m2/dose | 30 | mg/m2/dose | intravenous injection, IV | q3w | Doxorubicin Hydrochloride Liposome | doxorubicin hydrochloride liposome | PDR |