Basic Information
| Drug ID | DDPD00995 |

|
| Drug Name | Auranofin | |
| Molecular Weight | 678.48 | |
| Molecular Formula | C20H34AuO9PS | |
| CAS Number | 34031-32-8 | |
| SMILES | CC[P+](CC)(CC)[Au-]S[C@@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O | |
| External Links | ||
| DRUGBANK | DB00995 | |
| PubChem Compound | 70788951 | |
| PDR | 795 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
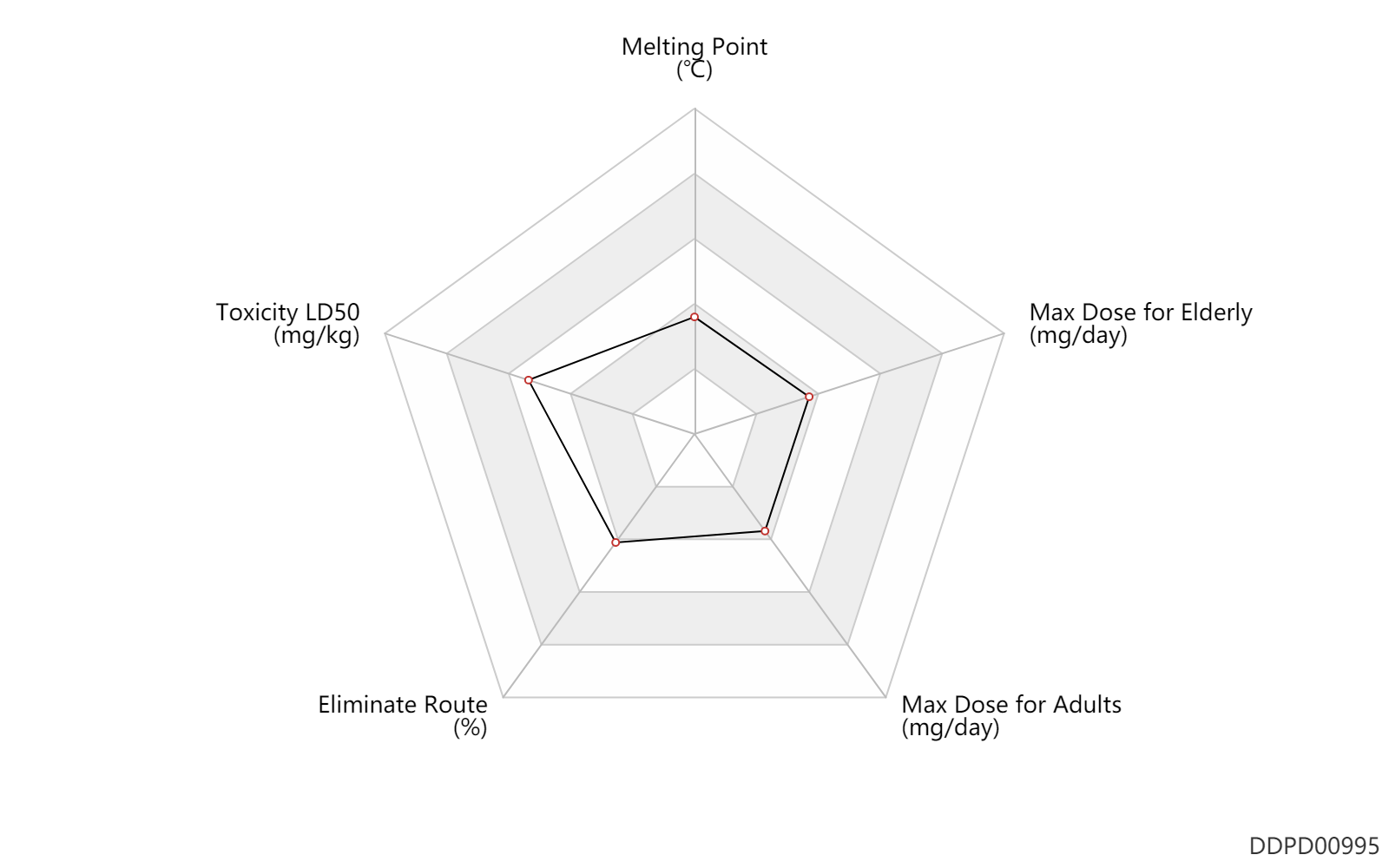
| Melting Point | 110.5 | ℃ | 110-111 | ℃ | McGusty, E.R. and Sutton, B.M.; U S . Patent 3,708,579; January 2, 1973; assigned to SmithKline and French Laboratories. Nemeth, P.E. and Sutton, B.M.; U.S. Patent 3,635,945; January 18, 1972; assigned to SmithKline and French Laboratories. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Toxicity LD50 | 2000.0 | mg/kg | >2000 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 15.0 | % | 15 | % | Urinary excretion; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 9.0 | mg/day | 9 | mg/day | PO, oral | Ridaura | auranofin | PDR |
| Max dose for elderly | 9.0 | mg/day | 9 | mg/day | PO, oral | Ridaura | auranofin | PDR |