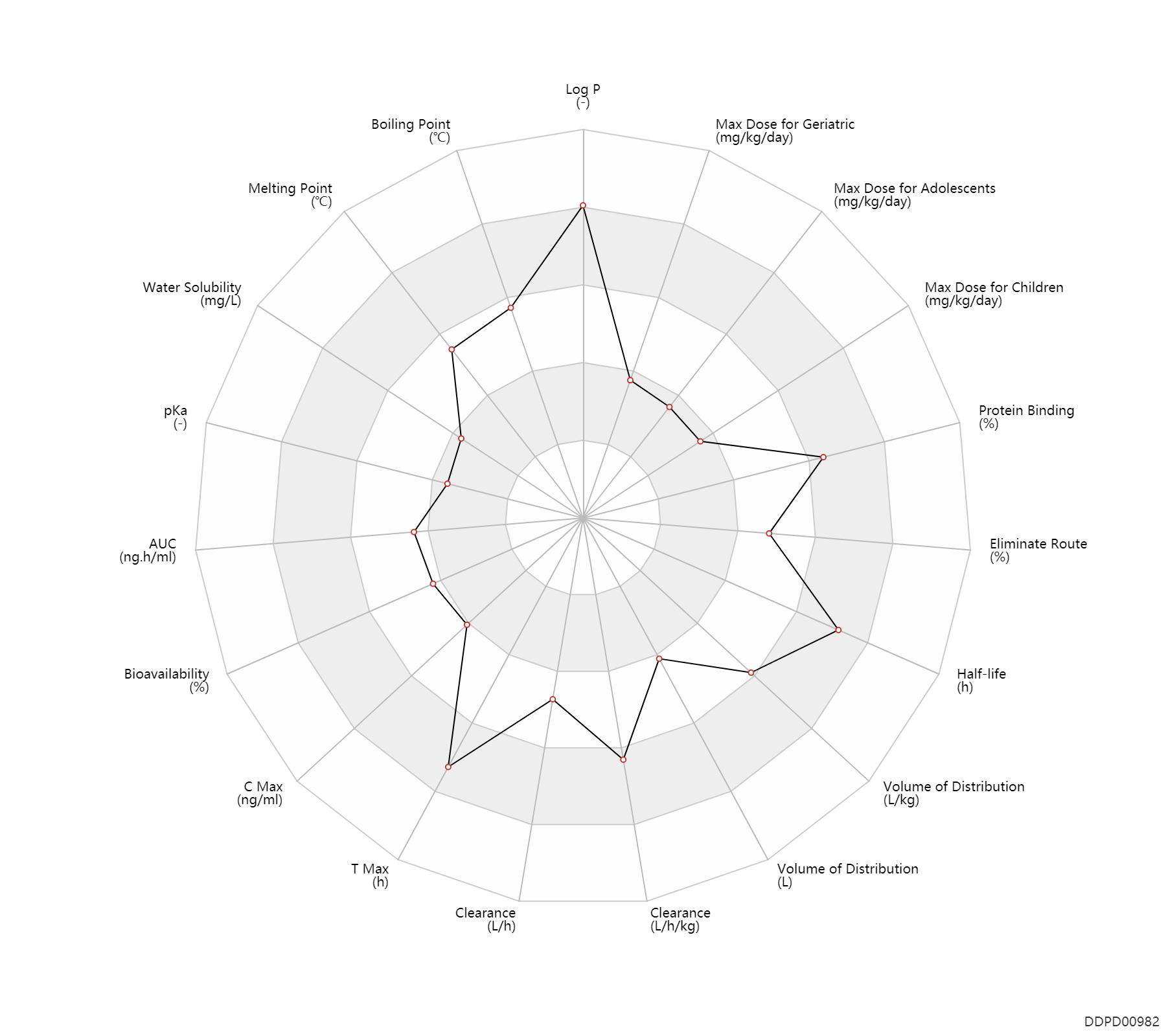
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| AUC |
4055.0 |
ng.h/ml |
4055.0 |
ng.h/ml |
PO, oral; fasting; |
DRUGBANK |
AUC |
6095.0 |
ng.h/ml |
6095.0 |
ng.h/ml |
PO, oral; high-fat meal; |
DRUGBANK |
| Bioavailability |
40.0 |
% |
40.0 |
% |
PO, oral; |
The Pharmacological Basis of Therapeutics |
| C Max |
292.5 |
ng/ml |
74-511 |
ng/ml |
PO, oral; |
DRUGBANK |
C Max |
314.0 |
ng/ml |
314 |
ng/ml |
PO, oral; fasting; |
DRUGBANK |
C Max |
395.0 |
ng/ml |
395 |
ng/ml |
PO, oral; high-fat meal; |
DRUGBANK |
C Max |
208.0 |
ng/ml |
208±92 |
ng/ml |
PO, oral; |
The Pharmacological Basis of Therapeutics |
C Max |
473.0 |
ng/ml |
473±171 |
ng/ml |
PO, oral; Active metabolite; |
The Pharmacological Basis of Therapeutics |
| T Max |
2.5 |
h |
1-4 |
h |
PO, oral; |
DRUGBANK |
T Max |
2.9 |
h |
2.9 |
h |
PO, oral; fasting; |
DRUGBANK |
T Max |
6.4 |
h |
6.4 |
h |
PO, oral; high-fat meal; |
DRUGBANK |
T Max |
4.5 |
h |
4.5±3.4 |
h |
PO, oral; |
The Pharmacological Basis of Therapeutics |
T Max |
6.8 |
h |
6.8±6.5 |
h |
PO, oral; Active metabolite; |
The Pharmacological Basis of Therapeutics |
| Clearance |
15.9 |
L/h |
15.9 |
L/h |
neuroblastoma; pediatric patients; |
DRUGBANK |
Clearance |
1.3 |
L/h/kg |
21.3 |
ml/min/kg |
guinea pigs; |
DRUGBANK |
Clearance |
0.43 |
L/h/kg |
7.2 |
ml/min/kg |
Rattus, Rat; Obesity; |
DRUGBANK |
Clearance |
0.33 |
L/h/kg |
5.5(0.9-11.1) |
ml/min/kg |
apparent clearance; |
The Pharmacological Basis of Therapeutics |
| Volume of Distribution |
85.0 |
L |
85.0 |
L |
neuroblastoma; pediatric patients; |
DRUGBANK |
Volume of Distribution |
2.4 |
L/kg |
2423.0 |
ml/kg |
guinea pigs; |
DRUGBANK |
Volume of Distribution |
1.7 |
L/kg |
1716.0 |
ml/kg |
Rattus, Rat; Obesity; |
DRUGBANK |
Volume of Distribution |
5.0 |
L/kg |
5(1-32) |
L/kg |
Apparent volume of distribution; |
The Pharmacological Basis of Therapeutics |
| Half-life |
23.0 |
h |
7-39 |
h |
|
DRUGBANK |
Half-life |
20.0 |
h |
20 |
h |
elimination half-life; |
DRUGBANK |
Half-life |
33.5 |
h |
17-50 |
h |
|
DRUGBANK |
Half-life |
25.0 |
h |
25 |
h |
elimination half-life; |
DRUGBANK |
Half-life |
17.0 |
h |
17(5-167) |
h |
|
The Pharmacological Basis of Therapeutics |
Half-life |
29.0 |
h |
29±6 |
h |
|
The Pharmacological Basis of Therapeutics |
| Eliminate Route |
63.5 |
% |
53-74 |
% |
Faeces excretion; PO, oral; Unchanged drug; |
DRUGBANK |
Eliminate Route |
0 |
% |
~0 |
% |
Urinary excretion; Unchanged drug; |
The Pharmacological Basis of Therapeutics |
| Protein Binding |
99.9 |
% |
>99.9 |
% |
|
DRUGBANK |
Protein Binding |
99.0 |
% |
>99 |
% |
|
The Pharmacological Basis of Therapeutics |