Basic Information
| Drug ID | DDPD00972 |

|
| Drug Name | Azelastine | |
| Molecular Weight | 381.898 | |
| Molecular Formula | C22H24ClN3O | |
| CAS Number | 58581-89-8 | |
| SMILES | CN1CCCC(CC1)N1N=C(CC2=CC=C(Cl)C=C2)C2=CC=CC=C2C1=O | |
| External Links | ||
| DRUGBANK | DB00972 | |
| T3DB | T3D4558 | |
| PubChem Compound | 2267 | |
| PDR | 2003 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
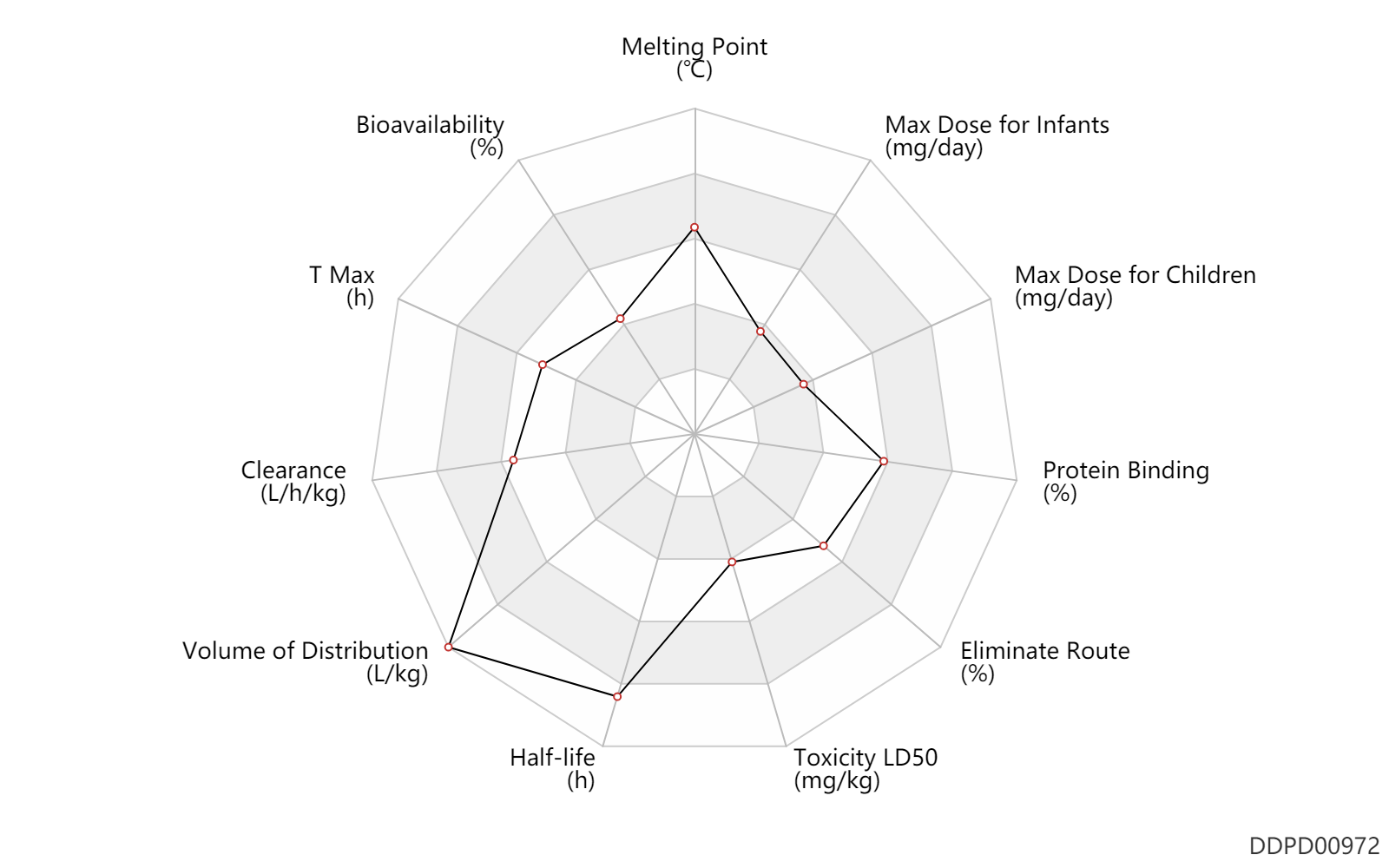
| Melting Point | 225.0 | ℃ | 225 | ℃ | FDA Label (intranasal azelastine) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 40.0 | % | 40 | % | inhalation, IH; | DRUGBANK |
| T Max | 2.5 | h | 2-3 | h | inhalation, IH; | DRUGBANK |
| Clearance | 0.50 | L/h/kg | 0.5 | l/h/kg | Plasma clearance; PO, oral; intravenous injection, IV; | DRUGBANK | Clearance | 0.54 | L/h/kg | 9 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 14.5 | L/kg | 14.5 | L/kg | Steady state volume of distribution; PO, oral; intravenous injection, IV; | DRUGBANK | Volume of Distribution | 15.0 | L/kg | 15 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 22.0 | h | 22 | h | elimination half-life; PO, oral; | DRUGBANK | Half-life | 54.0 | h | 54 | h | elimination half-life; | DRUGBANK | Half-life | 22.0 | h | 22 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 580.0 | mg/kg | 580.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 75.0 | % | ~75 | % | Faeces excretion; PO, oral; | DRUGBANK | Eliminate Route | 10.0 | % | <10 | % | PO, oral; Unchanged drug; | DRUGBANK |
| Protein Binding | 88.0 | % | ~88 | % | plasma proteins; high protein binding; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 0.274 | mg/day | 274 | mcg/day | nasal spray | bid | Optivar | azelastine hydrochloride | PDR |
| Max dose for children | 0.822 | mg/day | 822 | mcg/day | nasal spray | q6h | Optivar | azelastine hydrochloride | PDR |
| Max dose for children | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Optivar | azelastine hydrochloride | PDR | |
| Max dose for children | 0.274 | mg/day | 274 | mcg/day | nasal spray | bid | Optivar | azelastine hydrochloride | PDR |
| Max dose for children | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Optivar | azelastine hydrochloride | PDR | |
| Max dose for children | 0.274 | mg/day | 274 | mcg/day | nasal spray | bid | Optivar | azelastine hydrochloride | PDR |
| Max dose for children | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Optivar | azelastine hydrochloride | PDR | |
| Max dose for children | 0.274 | mg/day | 274 | mcg/day | nasal spray | bid | Optivar | azelastine hydrochloride | PDR |
| Max dose for adolescents | 4.0 | sprays/day/nostril | 4 | sprays/day/nostril | nasal spray | q6h | Optivar | azelastine hydrochloride | PDR |
| Max dose for adolescents | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Optivar | azelastine hydrochloride | PDR | |
| Max dose for adults | 4.0 | sprays/day/nostriL | 4 | sprays/day/nostriL | nasal spray | q6h | Optivar | azelastine hydrochloride | PDR |
| Max dose for adults | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Optivar | azelastine hydrochloride | PDR | |
| Max dose for geriatric | 4.0 | sprays/day/nostril | 4 | sprays/day/nostril | nasal spray | q6h | Optivar | azelastine hydrochloride | PDR |
| Max dose for geriatric | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Optivar | azelastine hydrochloride | PDR |