Basic Information
| Drug ID | DDPD00921 |

|
| Drug Name | Buprenorphine | |
| Molecular Weight | 467.6401 | |
| Molecular Formula | C29H41NO4 | |
| CAS Number | 52485-79-7 | |
| SMILES | CO[C@]12CC[C@@]3(C[C@@H]1[C@](C)(O)C(C)(C)C)[C@H]1CC4=C5C(O[C@@H]2[C@@]35CCN1CC1CC1)=C(O)C=C4 | |
| External Links | ||
| DRUGBANK | DB00921 | |
| T3DB | T3D2932 | |
| PubChem Compound | 644073 | |
| PDR | 1528 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
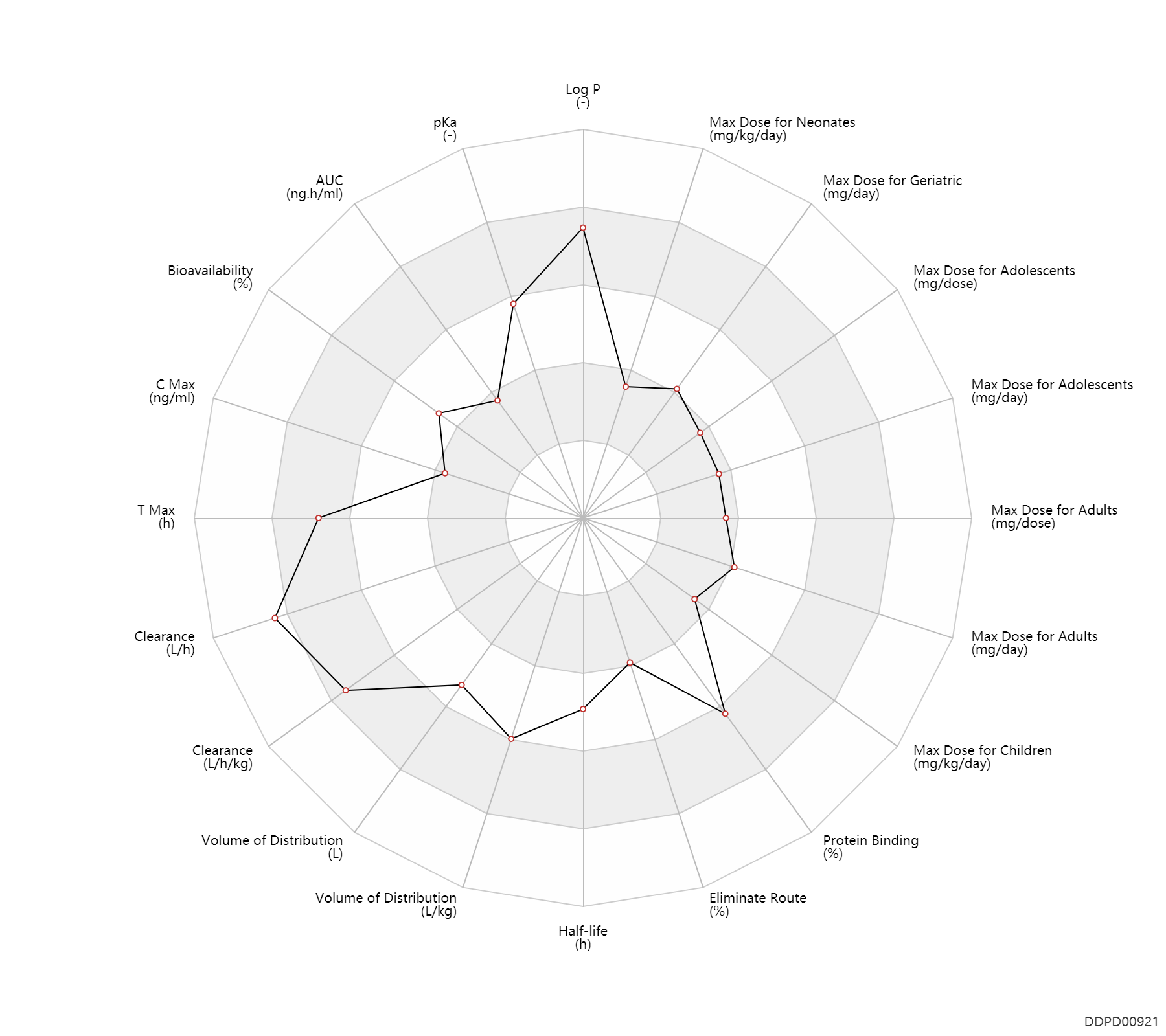
| Log P | 4.98 | - | 4.98 | - | AVDEEF,A ET AL. (1996) |
| pKa | 8.31 | - | 8.31 | - | AVDEEF,A ET AL. (1996) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| AUC | 7.7 | ng.h/ml | 7.651 | ng.h/ml | DRUGBANK | ||
| Bioavailability | 65.0 | % | 40->90 | % | IM,intramuscular injection; | The Pharmacological Basis of Therapeutics | Bioavailability | 51.0 | % | 51±30 | % | subcutaneous injection, SC; | The Pharmacological Basis of Therapeutics | Bioavailability | 28.0 | % | 28±9 | % | buccal; | The Pharmacological Basis of Therapeutics |
| C Max | 0.78 | ng/ml | 0.78 | ng/ml | DRUGBANK | C Max | 3.6 | ng/ml | 3.6±3.0 | ng/ml | IM,intramuscular injection; | The Pharmacological Basis of Therapeutics | C Max | 3.3 | ng/ml | 3.3±0.8 | ng/ml | subcutaneous injection, SC; | The Pharmacological Basis of Therapeutics | C Max | 2.0 | ng/ml | 2.0±0.6 | ng/ml | buccal; | The Pharmacological Basis of Therapeutics |
| T Max | 15.0 | h | 15 | h | DRUGBANK | T Max | 0.0800 | h | 0.08 | h | IM,intramuscular injection; | The Pharmacological Basis of Therapeutics | T Max | 0.70 | h | 0.7±0.1 | h | subcutaneous injection, SC; | The Pharmacological Basis of Therapeutics | T Max | 0.80 | h | 0.8±0.2 | h | buccal; | The Pharmacological Basis of Therapeutics |
| Clearance | 54.6 | L/h | 901.2±39.7 | ml/min | Plasma clearance; intravenous injection, IV; patients; | DRUGBANK | Clearance | 69.7 | L/h | 1042-1280 | ml/min | Plasma clearance; intravenous injection, IV; normal,healthy; | DRUGBANK | Clearance | 0.80 | L/h/kg | 13.3±0.59 | ml/min/kg | surgery; Male, men; Female, women; | Children ↑ ; | The Pharmacological Basis of Therapeutics | Clearance | 1.1 | L/h/kg | 19 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 261.5 | L | 188-335 | L | intravenous injection, IV; | DRUGBANK | Volume of Distribution | 1.4 | L/kg | 1.44±0.11 | L/kg | surgery; Male, men; Female, women; | Children ↑ ; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 4.9 | L/kg | 4.9 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 2.8 | h | ~166 | min | DRUGBANK | Half-life | 30.8 | h | 30.75 | h | Multiple dose; sublingual; | DRUGBANK | Half-life | 2.3 | h | 2.33±0.24 | h | Male, men; Female, women; surgery; | Children ↓ ; | The Pharmacological Basis of Therapeutics | Half-life | 3.2 | h | 3.2 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 20.0 | % | 10-30 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 0 | % | ~0 | % | Urinary excretion; patients; human, homo sapiens; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 96.0 | % | ~96 | % | DRUGBANK | Protein Binding | 96.0 | % | 96 | % | patients; human, homo sapiens; | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 0.06 | mg/kg/day | 60 | mcg/kg/day | sublingual | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for children | 0.024 | mg/kg/day | 6 | mcg/kg/dose | intravenous injection, IV;IM,intramuscular injection; | q4h-q8h | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR |
| Max dose for adolescents | 0.6 | mg/dose | 0.6 | mg/dose | IM,intramuscular injection | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adolescents | 0.3 | mg/dose | 0.3 | mg/dose | intravenous injection, IV | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adolescents | 1.62630136986301 | mg/day | 593.6 | mg/year | subdermal implant | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adolescents | 0.6 | mg/dose | 0.6 | mg/dose | IM,intramuscular injection | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adolescents | 0.3 | mg/dose | 0.3 | mg/dose | intravenous injection, IV | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adults | 0.6 | mg/dose | 0.6 | mg/dose | IM,intramuscular injection | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adults | 0.3 | mg/dose | 0.3 | mg/dose | intravenous injection, IV | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adults | 480.0 | mg/day | 20 | mg/h | Transdermal preparations | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adults | 1.8 | mg/day | 1800 | mcg/day | skin/dermal | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adults | 24.0 | mg/day | 24 | mg/day | sublingual | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adults | 10.0 | mg/day | 300 | mg/month | subcutaneous injection, SC | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for adults | 1.62630136986301 | mg/day | 593.6 | mg/year | subdermal implant | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for geriatric | 0.6 | mg/dose | 0.6 | mg/dose | IM,intramuscular injection | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for geriatric | 0.3 | mg/dose | 0.3 | mg/dose | intravenous injection, IV | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for geriatric | 480.0 | mg/day | 20 | mg/hour | Transdermal preparations | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for geriatric | 1.8 | mg/day | 1800 | mcg/day | skin/dermal | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for geriatric | 24.0 | mg/day | 24 | mg/day | sublingual | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for geriatric | 10.0 | mg/day | 300 | mg/month | subcutaneous injection, SC | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR | |
| Max dose for geriatric | 1.62630136986301 | mg/day | 593.6 | mg/year | subdermal implant | Buprenorphine Hydrochloride | buprenorphine hydrochloride | PDR |