Basic Information
| Drug ID | DDPD00915 |

|
| Drug Name | Amantadine | |
| Molecular Weight | 151.2487 | |
| Molecular Formula | C10H17N | |
| CAS Number | 768-94-5 | |
| SMILES | NC12CC3CC(CC(C3)C1)C2 | |
| External Links | ||
| DRUGBANK | DB00915 | |
| T3DB | T3D2560 | |
| PubChem Compound | 2130 | |
| PDR | 1475 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
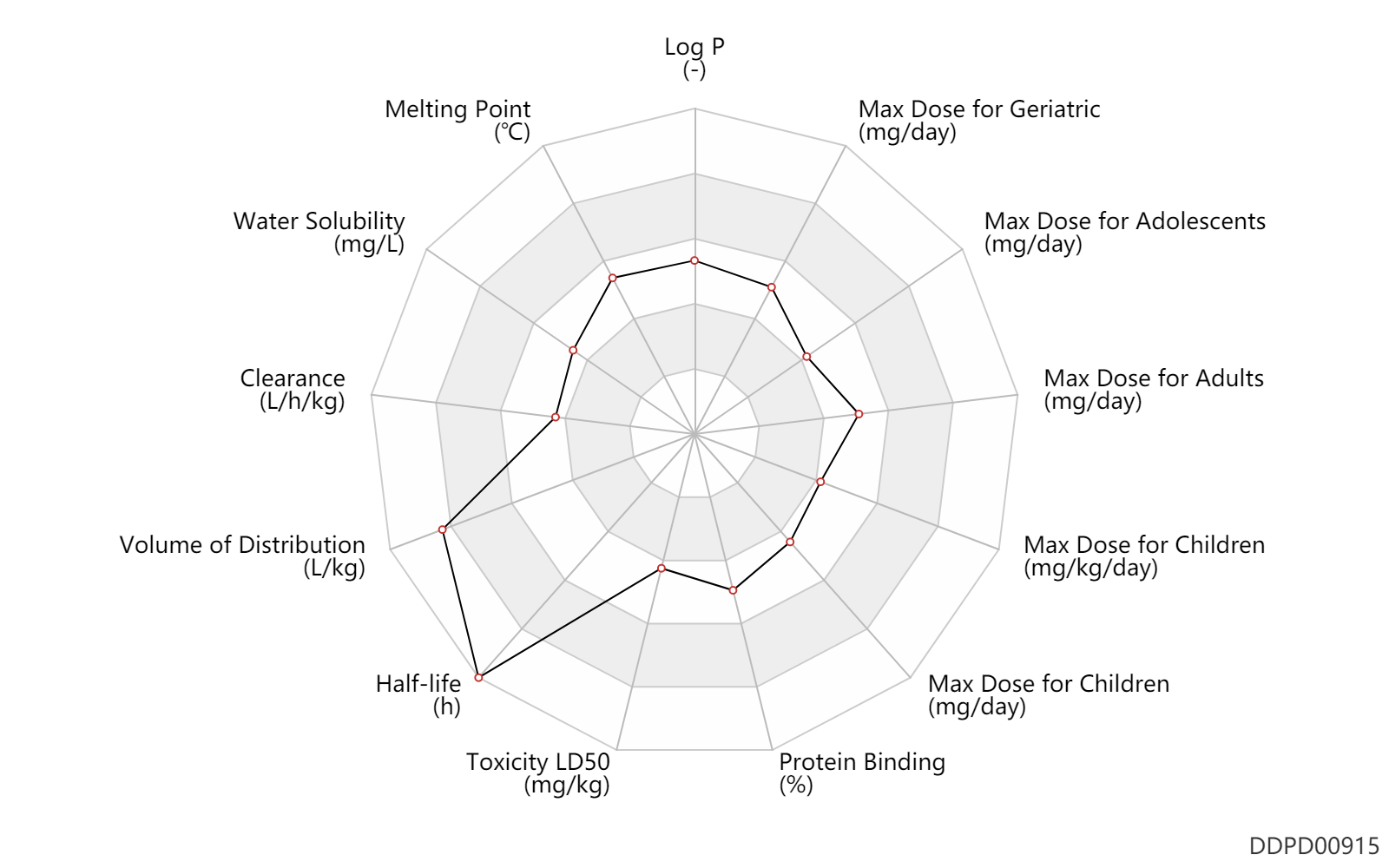
| Log P | 2.44 | - | 2.44 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 186.0 | ℃ | 180-192 | ℃ | Haaf, W.; U.S. Patent 3,152,180; October 6, 1964; assigned to Studiengesellschaft Kohle mbH, Germany. |
| Water Solubility | 6290.0 | mg/L | 6290 | mg/L | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Clearance | 0.25 | L/h/kg | 0.2-0.3 | L/h/kg | DRUGBANK | Clearance | 0.10 | L/h/kg | 0.10±0.04 | L/h/kg | normal,healthy; Elderly; Male, men; | DRUGBANK | Clearance | 0.29 | L/h/kg | 4.8 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 5.5 | L/kg | 3.0-8 | L/kg | normal,healthy; | DRUGBANK | Volume of Distribution | 6.6 | L/kg | 6.6 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 12.0 | h | 10-14 | h | DRUGBANK | Half-life | 204.0 | h | 7-10 | day | RD, renal impairment, Renal disease,including uremia; | DRUGBANK | Half-life | 16.0 | h | 16 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 800.0 | mg/kg | 800.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB | Toxicity LD50 | 700.0 | mg/kg | 700.0 | mg/kg | PO, oral; mouse; | T3DB |
| Toxicity Lethal Dose | 2000.0 | mg | 2.0 | g | T3DB | |
| Protein Binding | 67.0 | % | ~67 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 200.0 | mg/day | 200 | mg/day | Tablet,PO,oral;Capsule, PO, Oral;Liquid; | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for children | 8.8 | mg/kg/day | 8.8 | mg/kg/day | Tablet,PO,oral;Capsule, PO, Oral;Liquid; | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for children | 150.0 | mg/day | 150 | mg/day | Tablet,PO,oral;Capsule, PO, Oral;Liquid; | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for adolescents | 200.0 | mg/day | 200 | mg/day | Tablet,PO,oral;Capsule, PO, Oral;Liquid; | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for adults | 400.0 | mg/day | 400 | mg/day | Tablet,PO,oral;Capsule, PO, Oral;Liquid; | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for adults | 274.0 | mg/day | 274 | mg/day | Capsule, PO, Oral | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for adults | 322.0 | mg/day | 322 | mg/day | Tablet,PO,oral | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for geriatric | 400.0 | mg/day | 400 | mg/day | Tablet,PO,oral;Capsule, PO, Oral;Liquid; | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for geriatric | 274.0 | mg/day | 274 | mg/day | Capsule, PO, Oral | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |
| Max dose for geriatric | 322.0 | mg/day | 322 | mg/day | Tablet,PO,oral | Amantadine Hydrochloride Capsules | amantadine hydrochloride | PDR |