Basic Information
| Drug ID | DDPD00905 |

|
| Drug Name | Bimatoprost | |
| Molecular Weight | 415.5656 | |
| Molecular Formula | C25H37NO4 | |
| CAS Number | 155206-00-1 | |
| SMILES | CCNC(=O)CCC\C=C/C[C@H]1[C@@H](O)C[C@@H](O)[C@@H]1\C=C\[C@@H](O)CCC1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB00905 | |
| PubChem Compound | 5311027 | |
| PDR | 1715 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
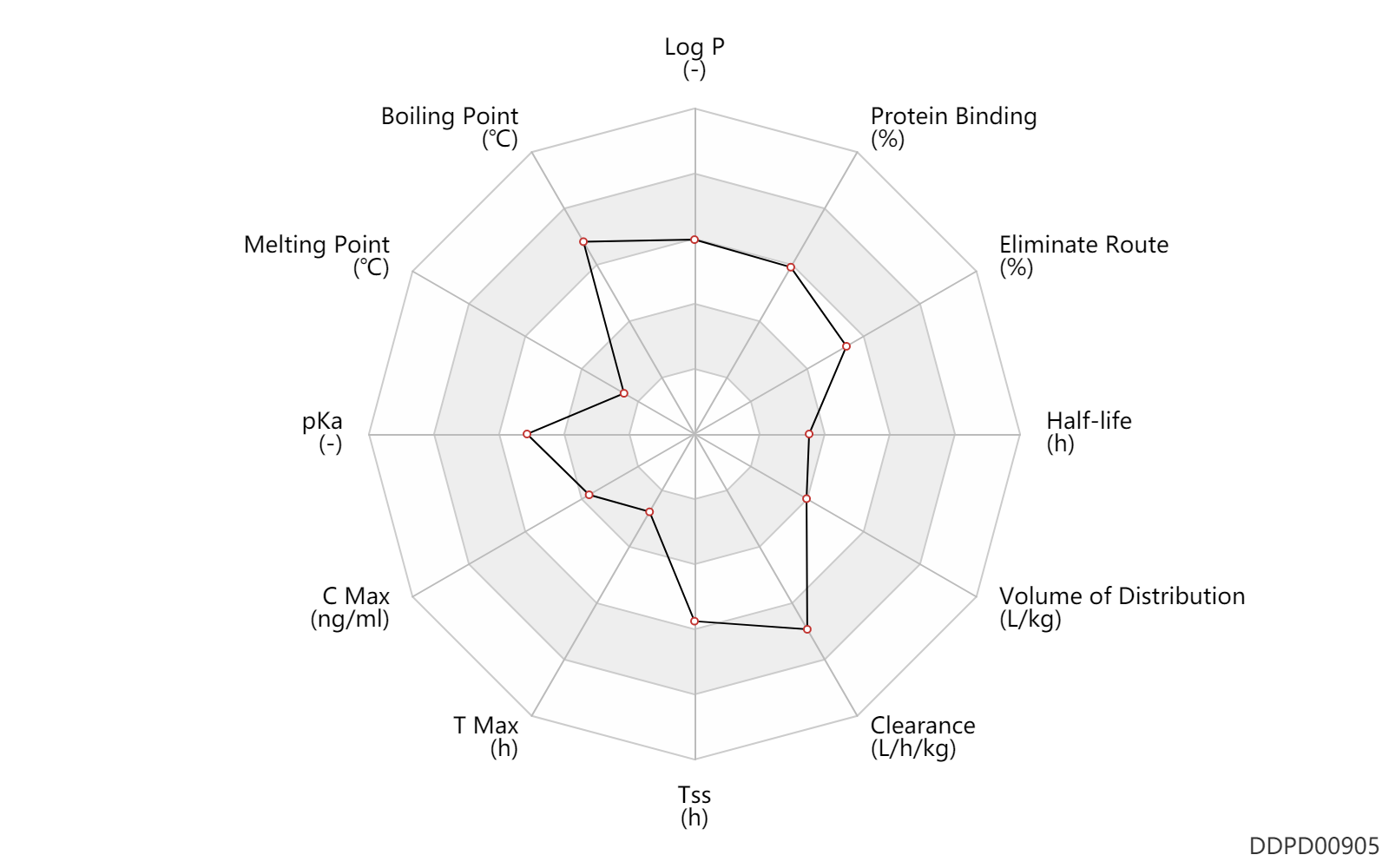
| Log P | 3.2 | - | 3.2 | - | http://www.hmdb.ca/metabolites/HMDB0015041 |
| Boiling Point | 629.8 | ℃ | 629.8 | ℃ | https://mri.cts-mrp.eu/Human/Downloads/PT_H_1188_001_PAR.pdf |
| Melting Point | 65.0 | ℃ | 63-67 | ℃ | https://mri.cts-mrp.eu/Human/Downloads/PT_H_1188_001_PAR.pdf |
| pKa | 14.3 | - | 14.3,-0.23 | - | http://www.hmdb.ca/metabolites/HMDB0015041 | pKa | -0.23 | - | 14.3,-0.23 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 0.0900 | null | 0.09 | null | ophthalmic administration; | DRUGBANK |
| C Max | 0.0800 | ng/ml | 0.08 | ng/ml | ophthalmic administration; | DRUGBANK | C Max | 12.2 | ng/ml | 12.2 | ng/ml | different study; | DRUGBANK |
| T Max | 0.17 | h | 10 | min | different study; | DRUGBANK |
| Tss | 168.0 | h | 7 | day | different study; | DRUGBANK |
| Clearance | 0.15 | L/h/kg | 0.15 | L/h/kg | intravenous injection, IV; normal,healthy; | DRUGBANK | Clearance | 1.5 | L/h/kg | 25 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.67 | L/kg | 0.67 | L/kg | at steady state; | DRUGBANK | Volume of Distribution | 0.67 | L/kg | 0.67 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 0.75 | h | ~45 | min | elimination half-life; | DRUGBANK | Half-life | 0.45 | h | 0.45 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 67.0 | % | 67 | % | Urinary excretion; normal,healthy; human, homo sapiens; | DRUGBANK | Eliminate Route | 25.0 | % | 25 | % | Faeces excretion; normal,healthy; human, homo sapiens; | DRUGBANK |
| Protein Binding | 89.0 | % | ~88-90 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for children | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | qd | Latisse | bimatoprost | PDR |
| Max dose for adolescents | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | qd | Latisse | bimatoprost | PDR |
| Max dose for adults | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | qd | Latisse | bimatoprost | PDR |
| Max dose for geriatric | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | qd | Latisse | bimatoprost | PDR |