Basic Information
| Drug ID | DDPD00898 |

|
| Drug Name | Ethanol | |
| Molecular Weight | 46.0684 | |
| Molecular Formula | C2H6O | |
| CAS Number | 64-17-5 | |
| SMILES | CCO | |
| External Links | ||
| DRUGBANK | DB00898 | |
| T3DB | T3D0770 | |
| PubChem Compound | 702 | |
| PDR | 3100 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
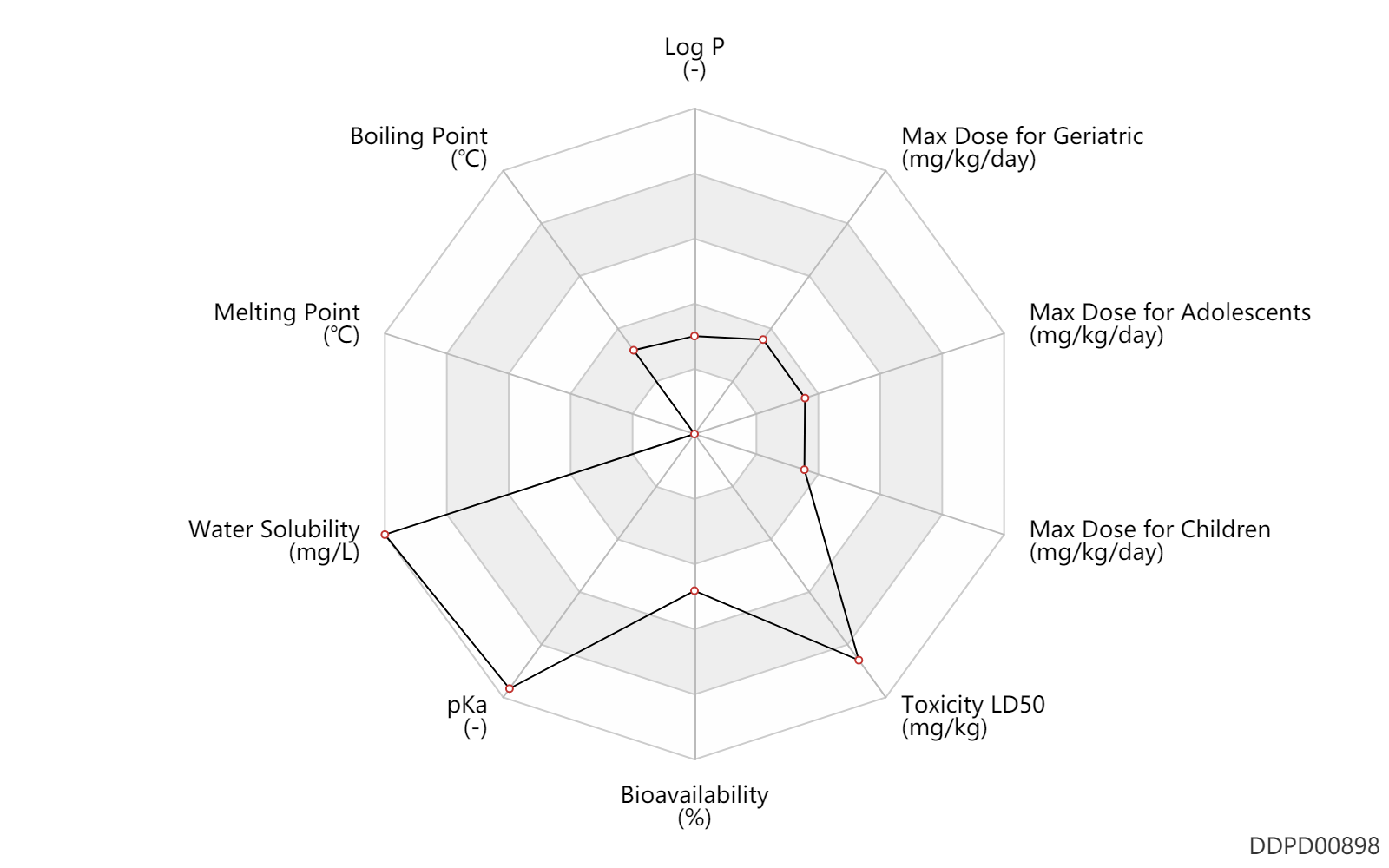
| Log P | -0.31 | - | -0.31 | - | HANSCH,C ET AL. (1995) |
| Boiling Point | 78.2 | ℃ | 78.2 | ℃ | PhysProp |
| Melting Point | -114.1 | ℃ | -114.1 | ℃ | PhysProp |
| Water Solubility | 1000000.0 | mg/L | 1000000 | mg/L | RIDDICK,JA ET AL. 1986) |
| pKa | 15.9 | - | 15.9 | - | RIDDICK,JA ET AL. (1986) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 53.0 | % | 53 | % | sublingual; | DRUGBANK |
| Toxicity LD50 | 5628.0 | mg/kg | 5628.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 5628.0 | mg/kg | 5628.0 | mg/kg | PO, oral; Rattus, Rat; | T3DB |
| Toxicity Lethal Dose | 350.0 | ml | 300-400 | ml | adults; human, homo sapiens; | T3DB | Toxicity Lethal Dose | 700.0 | ml | 600-800 | ml | adults; human, homo sapiens; | T3DB | Toxicity Lethal Dose | 6500.0 | mg/kg | 5-8 | g/kg | adults; human, homo sapiens; | T3DB | Toxicity Lethal Dose | 3000.0 | mg/kg | 3.0 | g/kg | Children; | T3DB |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 0.05 | mg/kg/day | 0.05 | mg/kg/day | subcutaneous injection, SC | Gattex | teduglutide (rDNA origin) | PDR |
| Max dose for adolescents | 0.05 | mg/kg/day | 0.05 | mg/kg/day | subcutaneous injection, SC | Gattex | teduglutide (rDNA origin) | PDR |
| Max dose for adults | 0.05 | mg/kg/day | 0.05 | mg/kg/day | subcutaneous injection, SC | Gattex | teduglutide (rDNA origin) | PDR |
| Max dose for geriatric | 0.05 | mg/kg/day | 0.05 | mg/kg/day | subcutaneous injection, SC | Gattex | teduglutide (rDNA origin) | PDR |