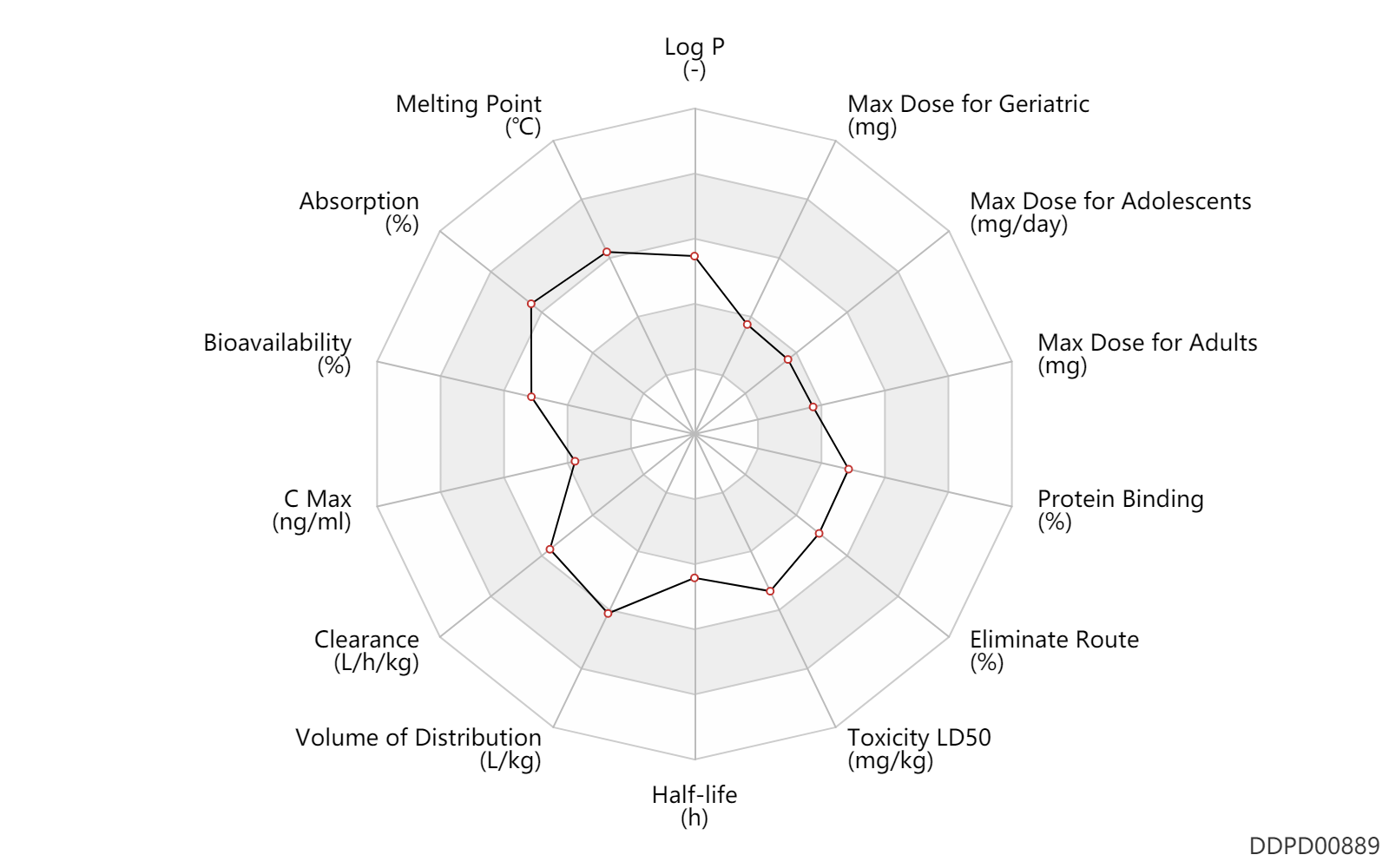
| Max dose for children |
0.01 |
mg/kg |
10 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for children |
0.04 |
mg/kg |
40 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for children |
0.04 |
mg/kg |
40 |
mcg/kg |
PO, oral |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for adolescents |
0.01 |
mg/day |
10 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for adolescents |
0.04 |
mg/day |
40 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for adolescents |
0.04 |
mg/day |
40 |
mcg/kg |
PO, oral |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for adults |
0.04 |
mg/kg |
40 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for adults |
2.0 |
mg |
2 |
mg |
PO, oral |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for adults |
1.0 |
patch |
1 |
patch |
skin/dermal |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for adults |
10.0 |
mg |
10 |
mg |
subcutaneous injection, SC |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for geriatric |
40.0 |
mcg/kg |
40 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for geriatric |
2.0 |
mg |
2 |
mg |
PO, oral |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for geriatric |
1.0 |
patch |
1 |
patch |
skin/dermal |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for geriatric |
10.0 |
mg |
10 |
mg |
subcutaneous injection, SC |
Granisetron Hydrochloride Tablets |
granisetron hydrochloride |
PDR |
| Max dose for children |
0.01 |
mg/kg |
10 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for children |
0.04 |
mg/kg |
40 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for children |
0.04 |
mg/kg |
40 |
mcg/kg |
PO, oral |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for adolescents |
0.01 |
mg/day |
10 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for adolescents |
0.04 |
mg/day |
40 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for adolescents |
0.04 |
mg/day |
40 |
mcg/kg |
PO, oral |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for adults |
0.04 |
mg/kg |
40 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for adults |
2.0 |
mg |
2 |
mg |
PO, oral |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for adults |
1.0 |
patch |
1 |
patch |
skin/dermal |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for adults |
10.0 |
mg |
10 |
mg |
subcutaneous injection, SC |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for geriatric |
40.0 |
mcg/kg |
40 |
mcg/kg |
intravenous injection, IV |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for geriatric |
2.0 |
mg |
2 |
mg |
PO, oral |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for geriatric |
1.0 |
patch |
1 |
patch |
skin/dermal |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |
| Max dose for geriatric |
10.0 |
mg |
10 |
mg |
subcutaneous injection, SC |
Granisetron Hydrochloride Injection 1 mg/mL and 0.1 mg/mL |
granisetron hydrochloride |
PDR |