Basic Information
| Drug ID | DDPD00887 |

|
| Drug Name | Bumetanide | |
| Molecular Weight | 364.416 | |
| Molecular Formula | C17H20N2O5S | |
| CAS Number | 28395-03-1 | |
| SMILES | CCCCNC1=C(OC2=CC=CC=C2)C(=CC(=C1)C(O)=O)S(N)(=O)=O | |
| External Links | ||
| DRUGBANK | DB00887 | |
| PubChem Compound | 2471 | |
| PDR | 1535 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
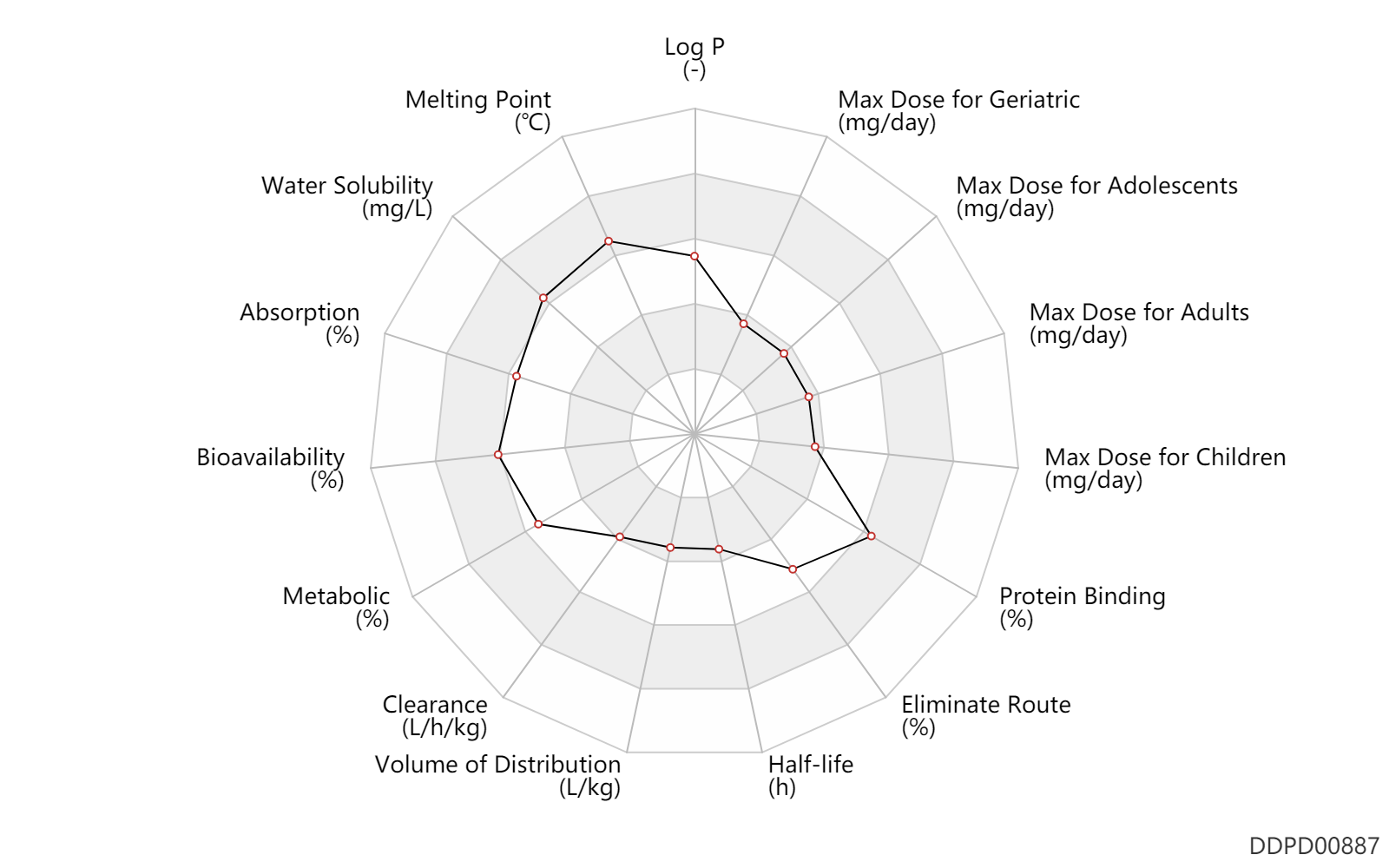
| Log P | 2.6 | - | 2.6 | - | DRUGBANK |
| Melting Point | 230.5 | ℃ | 230-231 | ℃ | Felt, P.W.; US. Patent 3,634,583; January 11, 1972; assigned to Lovens Kemiske Fabrik Produktionsaktieselskab, Denmark. |
| Water Solubility | 20000.0 | mg/L | >20 | mg/ml | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 80.0 | % | 80 | % | PO, oral; food; | food → ; | DRUGBANK |
| Bioavailability | 80.0 | % | ~80 | % | PO, oral; food; | food → ; | DRUGBANK |
| Metabolic | 45.0 | % | 45 | % | Unchanged drug; | DRUGBANK | |
| Clearance | 0.0390 | L/h/kg | 0.2-1.1 | ml/min/kg | Prem, premature; respiratory disorders; | DRUGBANK | Clearance | 0.13 | L/h/kg | 2.17 | ml/min/kg | overdose; Neonates; | DRUGBANK | Clearance | 0.11 | L/h/kg | 1.8±0.3 | ml/min/kg | Elderly; | DRUGBANK | Clearance | 0.17 | L/h/kg | 2.9±0.2 | ml/min/kg | young; | DRUGBANK | Clearance | 0.15 | L/h/kg | 2.5 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.16 | L/kg | 0.16 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset | |
| Half-life | 1.3 | h | 60-90 | min | DRUGBANK | Half-life | 1.2 | h | 1.2 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 81.0 | % | 81 | % | Urinary excretion; PO, oral; human, homo sapiens; | DRUGBANK | Eliminate Route | 2.0 | % | 2 | % | Bile excretion; PO, oral; human, homo sapiens; | DRUGBANK | Eliminate Route | 36.5 | % | 36.45 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 97.0 | % | 97 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 0.06 | mg/kg/dose | 0.06 | mg/kg/dose | intravenous injection, IV | Bumetanide Injection | bumetanide | PDR |
| Max dose for infants | 0.1 | mg/kg/dose | 0.1 | mg/kg/dose | intravenous injection, IV;PO, oral;IM,intramuscular injection; | Bumetanide Injection | bumetanide | PDR |
| Max dose for children | 0.1 | mg/kg/dose | 0.1 | mg/kg/dose | intravenous injection, IV;PO, oral;IM,intramuscular injection; | Bumetanide Injection | bumetanide | PDR |
| Max dose for children | 10.0 | mg/day | 10 | mg/day | intravenous injection, IV;PO, oral;IM,intramuscular injection; | Bumetanide Injection | bumetanide | PDR |
| Max dose for adolescents | 0.1 | mg/kg/dose | 0.1 | mg/kg/dose | intravenous injection, IV;PO, oral;IM,intramuscular injection; | Bumetanide Injection | bumetanide | PDR |
| Max dose for adolescents | 10.0 | mg/day | 10 | mg/day | intravenous injection, IV;PO, oral;IM,intramuscular injection; | Bumetanide Injection | bumetanide | PDR |
| Max dose for adults | 10.0 | mg/day | 10 | mg/day | intravenous injection, IV;PO, oral;IM,intramuscular injection; | Bumetanide Injection | bumetanide | PDR |
| Max dose for geriatric | 10.0 | mg/day | 10 | mg/day | intravenous injection, IV;PO, oral;IM,intramuscular injection; | Bumetanide Injection | bumetanide | PDR |