Basic Information
| Drug ID | DDPD00884 |

|
| Drug Name | Risedronic acid | |
| Molecular Weight | 283.1123 | |
| Molecular Formula | C7H11NO7P2 | |
| CAS Number | 105462-24-6 | |
| SMILES | OC(CC1=CN=CC=C1)(P(O)(O)=O)P(O)(O)=O | |
| External Links | ||
| DRUGBANK | DB00884 | |
| T3DB | T3D2668 | |
| PubChem Compound | 5245 | |
| PDR | 2511 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
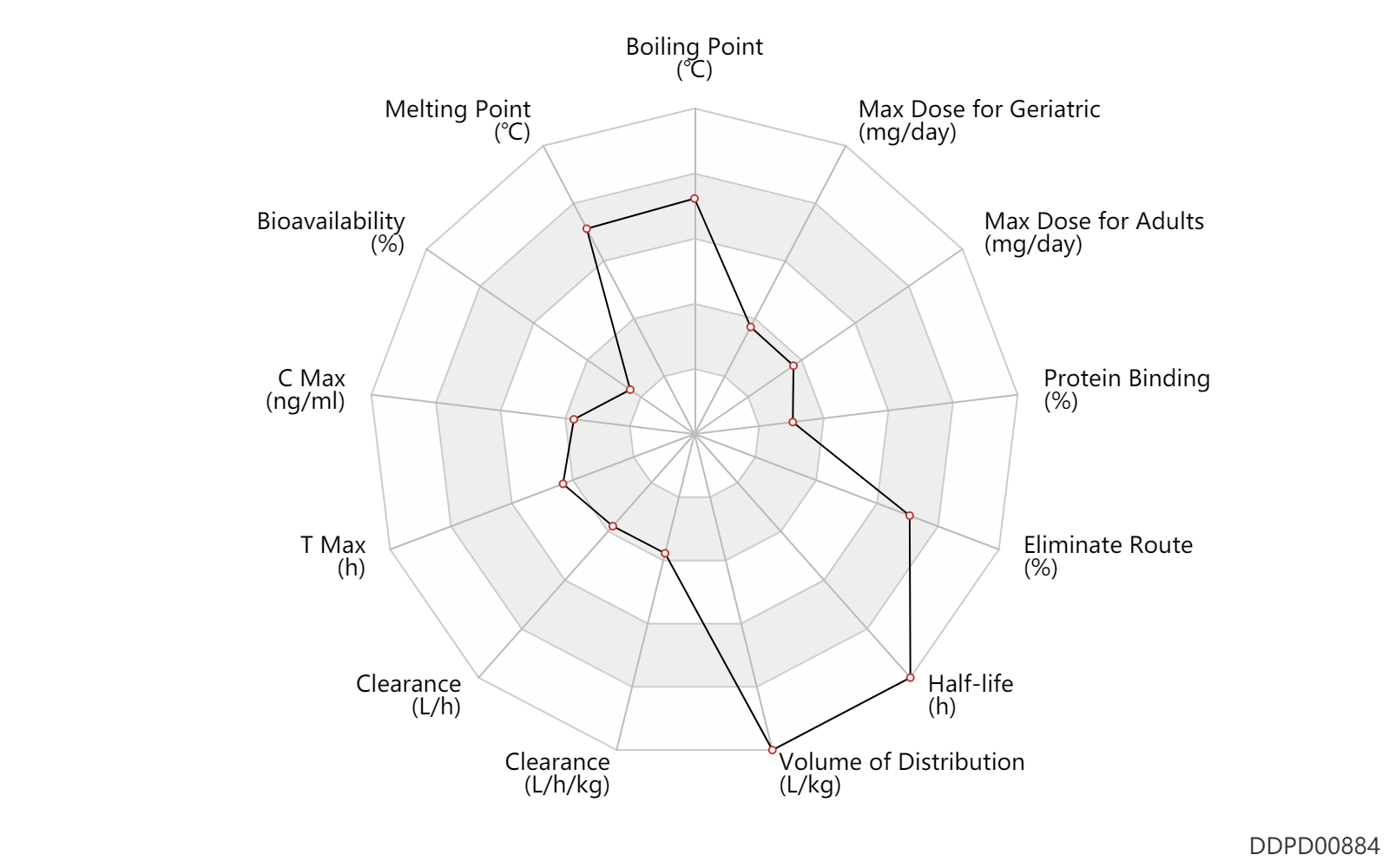
| Boiling Point | 692.3 | ℃ | 692.3 | ℃ | https://www.greenstonellc.com/sites/default/files/pdfs/MSDS/RISEDRONATE%20SODIUM%20TABLETS%20MSDS_0.pdf |
| Melting Point | 257.0 | ℃ | 252-262 | ℃ | https://www.greenstonellc.com/sites/default/files/pdfs/MSDS/RISEDRONATE%20SODIUM%20TABLETS%20MSDS_0.pdf |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 0.63 | % | 0.63 | % | PO, oral; | DRUGBANK | Bioavailability | 1.0 | % | <1 | % | PO, oral; | The Pharmacological Basis of Therapeutics |
| C Max | 2.8 | ng/ml | 2.8±0.3 | ng/ml | PO, oral; Male, men; normal,healthy; | The Pharmacological Basis of Therapeutics | |
| T Max | 1.7 | h | 1.7±1.2 | h | PO, oral; Male, men; normal,healthy; | The Pharmacological Basis of Therapeutics | |
| Clearance | 3.1 | L/h | 52.0 | ml/min | Renal clearance; | DRUGBANK | Clearance | 4.4 | L/h | 73.0 | ml/min | Average clearance; | DRUGBANK | Clearance | 0.0900 | L/h/kg | 1.5(1.2-1.9) | ml/min/kg | RD, renal impairment, Renal disease,including uremia ↓ ; | The Pharmacological Basis of Therapeutics |
| Volume of Distribution | 13.8 | L/kg | 13.8 | L/kg | DRUGBANK | Volume of Distribution | 6.3 | L/kg | 6.3±1.5 | L/kg | The Pharmacological Basis of Therapeutics |
| Half-life | 1.5 | h | ~1.5 | h | elimination half-life; | DRUGBANK | Half-life | 561.0 | h | 561 | h | terminal half-life; | DRUGBANK | Half-life | 200.0 | h | 200(183-218) | h | The Pharmacological Basis of Therapeutics | Half-life | 0.90 | h | 0.9 | h | distribution half-life; | The Pharmacological Basis of Therapeutics | Half-life | 13.0 | h | 13 | h | elimination half-life; | The Pharmacological Basis of Therapeutics |
| Eliminate Route | 87.0 | % | 87(73-102) | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics | |
| Protein Binding | 24.0 | % | ~24 | % | DRUGBANK | Protein Binding | 24.0 | % | 24 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 5.0 | mg/day | 5 | mg/day | PO, oral | Actonel | risedronate sodium | PDR |
| Max dose for adults | 5.0 | mg/day | 35 | mg/week | PO, oral | Actonel | risedronate sodium | PDR |
| Max dose for adults | 5.0 | mg/day | 150 | mg/month | PO, oral | Actonel | risedronate sodium | PDR |
| Max dose for adults | 30.0 | mg/day | 30 | mg/day | PO, oral | Actonel | risedronate sodium | PDR |
| Max dose for geriatric | 5.0 | mg/day | 5 | mg/day | PO, oral | Actonel | risedronate sodium | PDR |
| Max dose for geriatric | 5.0 | mg/day | 35 | mg/week | PO, oral | Actonel | risedronate sodium | PDR |
| Max dose for geriatric | 5.0 | mg/day | 150 | mg/month | PO, oral | Actonel | risedronate sodium | PDR |
| Max dose for geriatric | 30.0 | mg/day | 30 | mg/day | PO, oral | Actonel | risedronate sodium | PDR |