Basic Information
| Drug ID | DDPD00880 |

|
| Drug Name | Chlorothiazide | |
| Molecular Weight | 295.723 | |
| Molecular Formula | C7H6ClN3O4S2 | |
| CAS Number | 58-94-6 | |
| SMILES | NS(=O)(=O)C1=C(Cl)C=C2NC=NS(=O)(=O)C2=C1 | |
| External Links | ||
| DRUGBANK | DB00880 | |
| PubChem Compound | 2720 | |
| PDR | 1026 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
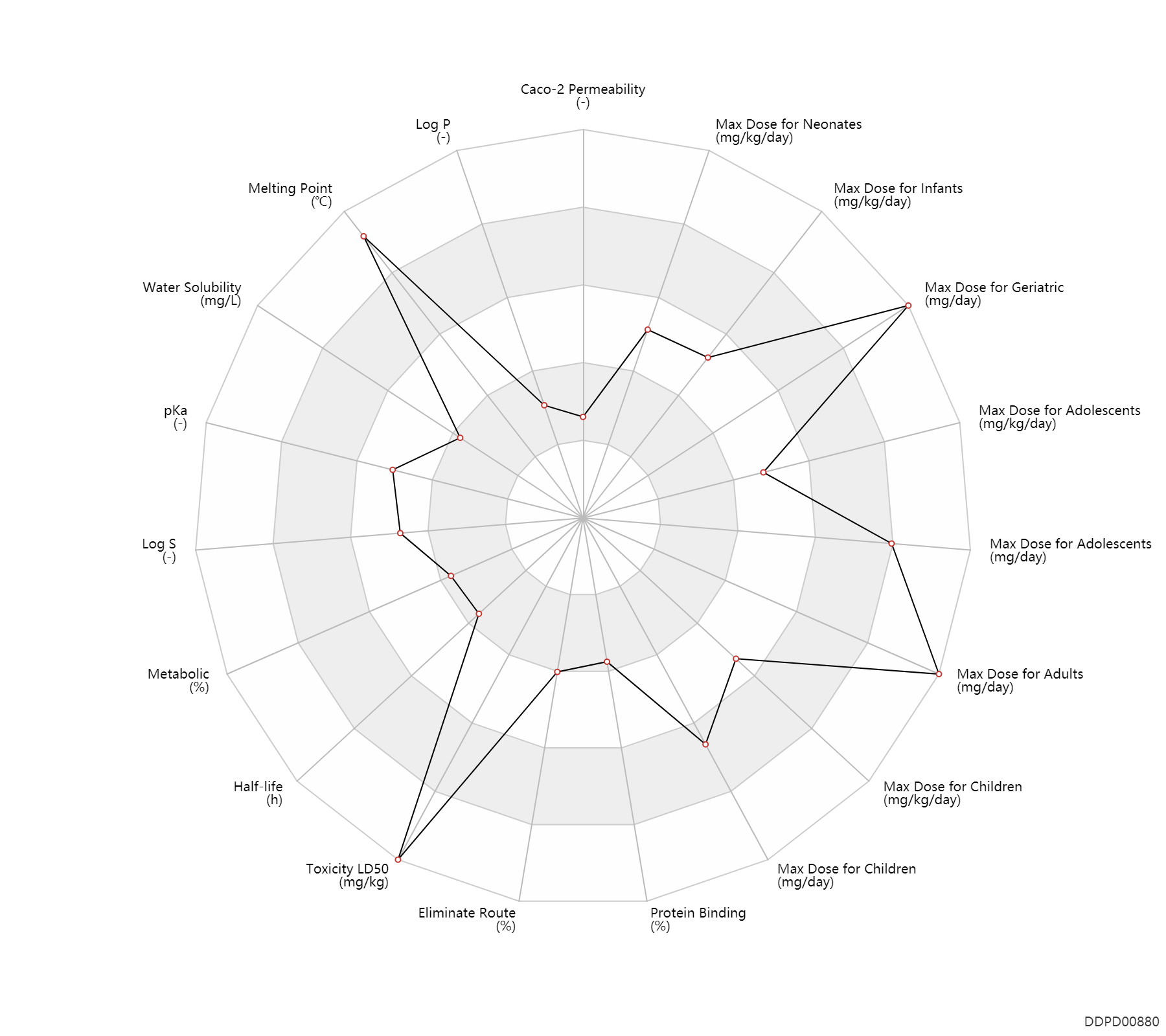
| Caco-2 Permeability | -6.72 | - | -6.72 | - | ADME Research, USCD |
| Log P | -0.24 | - | -0.24 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 342.75 | ℃ | 342.5-343 | ℃ | Novello, F.C.; US. Patent 2,809,194; October 8,1957; assigned to Merck & Co.,Inc. Hinkley, D.F.; US. Patent 2,937,169; May 17,1960; assigned to Merck & Co., Inc. |
| Water Solubility | 266.0 | mg/L | 266 | mg/L | YALKOWSKY,SH & DANNENFELSER,RM 1992) |
| pKa | 6.85 | - | 6.85 | - | MERCK INDEX (1996) |
| Log S | -3.05 | - | -3.05 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Metabolic | 0 | % | 0 | % | DRUGBANK | |
| Half-life | 1.4 | h | 45-120 | min | DRUGBANK | |
| Toxicity LD50 | 10000.0 | mg/kg | >10 | g/kg | PO, oral; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 12.5 | % | 10-15 | % | Urinary excretion; PO, oral; Unchanged drug; | DRUGBANK |
| Protein Binding | 40.0 | % | ~40 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 30.0 | mg/kg/day | 30 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for neonates | 40.0 | mg/kg/day | 40 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for infants | 20.0 | mg/kg/day | 20 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for infants | 40.0 | mg/kg/day | 40 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for infants | 20.0 | mg/kg/day | 20 | mg/kg/day | intravenous injection, IV | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for infants | 30.0 | mg/kg/day | 30 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for infants | 40.0 | mg/kg/day | 40 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for infants | 20.0 | mg/kg/day | 20 | mg/kg/day | intravenous injection, IV | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for children | 20.0 | mg/kg/day | 20 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for children | 1000.0 | mg/day | 1000 | mg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for children | 40.0 | mg/kg/day | 40 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for children | 20.0 | mg/kg/day | 20 | mg/kg/day | intravenous injection, IV | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for children | 20.0 | mg/kg/day | 20 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for children | 375.0 | mg/day | 375 | mg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for children | 40.0 | mg/kg/day | 40 | mg/kg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for children | 20.0 | mg/kg/day | 20 | mg/kg/day | intravenous injection, IV | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for adolescents | 2000.0 | mg/day | 2000 | mg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for adolescents | 1000.0 | mg/day | 1000 | mg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for adolescents | 20.0 | mg/kg/day | 20 | mg/kg/day | intravenous injection, IV | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for adolescents | 2000.0 | mg/day | 2000 | mg/day | intravenous injection, IV | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for adults | 2000.0 | mg/day | 2000 | mg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for adults | 1000.0 | mg/day | 1000 | mg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for adults | 2000.0 | mg/day | 2000 | mg/day | intravenous injection, IV | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for geriatric | 2000.0 | mg/day | 2000 | mg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for geriatric | 1000.0 | mg/day | 1000 | mg/day | PO, oral | Diuril Oral Suspension | chlorothiazide | PDR |
| Max dose for geriatric | 2000.0 | mg/day | 2000 | mg/day | intravenous injection, IV | Diuril Oral Suspension | chlorothiazide | PDR |