Basic Information
| Drug ID | DDPD00879 |

|
| Drug Name | Emtricitabine | |
| Molecular Weight | 247.247 | |
| Molecular Formula | C8H10FN3O3S | |
| CAS Number | 143491-57-0 | |
| SMILES | NC1=NC(=O)N(C=C1F)[C@@H]1CS[C@H](CO)O1 | |
| External Links | ||
| DRUGBANK | DB00879 | |
| PubChem Compound | 60877 | |
| PDR | 2517 | |
| Drugs.com | Drugs.com Drug Page | |
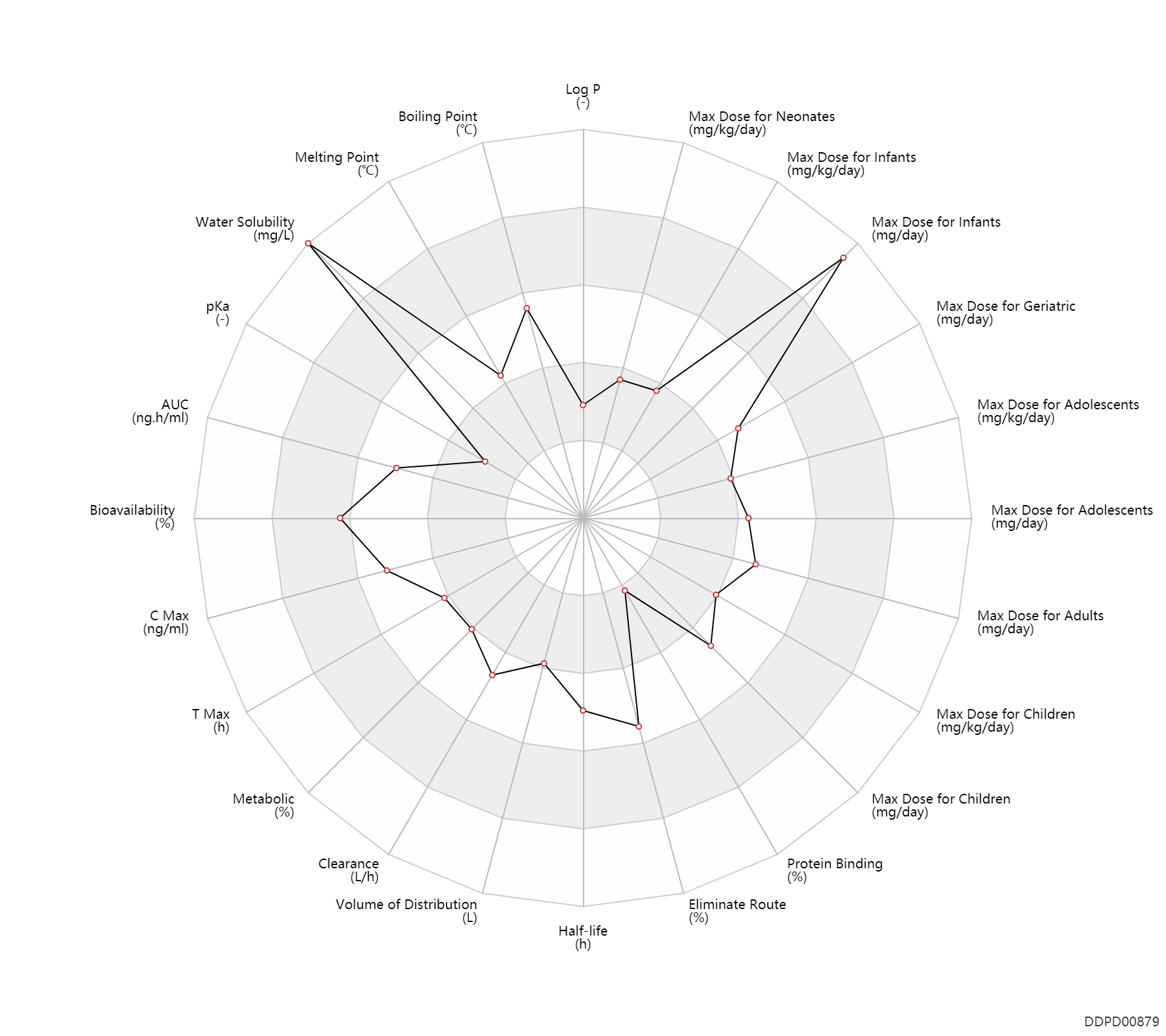
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | -0.43 | - | -0.43 | - | FDA Label |
| Boiling Point | 443.28 | ℃ | 443.28 | ℃ | ChemSpider |
| Melting Point | 137.0 | ℃ | 136-138 | ℃ | ChemSpider |
| Water Solubility | 112000.0 | mg/L | 112 | mg/ml | FDA Label |
| pKa | 2.65 | - | 2.65 | - | FDA Label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| AUC | 10000.0 | ng.h/ml | 10±3.1 | ug.h/ml | DRUGBANK | ||
| Bioavailability | 93.0 | % | 93 | % | Tablet, PO, oral; | DRUGBANK | Bioavailability | 75.0 | % | 75 | % | Capsule, PO, Oral; | DRUGBANK |
| C Max | 1800.0 | ng/ml | 1.8±0.7 | ug/ml | DRUGBANK | C Max | 1278.0 | ng/ml | 1.278±0.497 | ug/ml | food; | food ↓ ; | DRUGBANK |
| T Max | 1.5 | h | 1-2 | h | DRUGBANK | ||
| Metabolic | 14.0 | % | 14 | % | DRUGBANK | Metabolic | 9.0 | % | 9 | % | DRUGBANK | Metabolic | 2.0 | % | 2 | % | DRUGBANK |
| Clearance | 15.1 | L/h | 15.1 | L/h | DRUGBANK | ||
| Volume of Distribution | 42.3 | L | 42.3 | L | Total volume of distribution; | DRUGBANK | Volume of Distribution | 55.4 | L | 55.4 | L | Total volume of distribution; | DRUGBANK |
| Half-life | 10.0 | h | ~10 | h | DRUGBANK | ||
| Eliminate Route | 86.0 | % | 86 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 14.0 | % | 14 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 4.0 | % | <4 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 3.0 | mg/kg/day | 3 | mg/kg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for infants | 6.0 | mg/kg/day | 6 | mg/kg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for infants | 240.0 | mg/day | 240 | mg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for infants | 3.0 | mg/kg/day | 3 | mg/kg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for children | 200.0 | mg/day | 200 | mg/day | Capsule, PO, Oral | Emtriva | emtricitabine | PDR |
| Max dose for children | 6.0 | mg/kg/day | 6 | mg/kg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for children | 240.0 | mg/day | 240 | mg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for children | 6.0 | mg/kg/day | 6 | mg/kg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for children | 240.0 | mg/day | 240 | mg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for adolescents | 200.0 | mg/day | 200 | mg/day | Capsule, PO, Oral | Emtriva | emtricitabine | PDR |
| Max dose for adolescents | 6.0 | mg/kg/day | 6 | mg/kg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for adolescents | 240.0 | mg/day | 240 | mg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for adolescents | 6.0 | mg/kg/day | 6 | mg/kg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for adolescents | 240.0 | mg/day | 240 | mg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for adults | 200.0 | mg/day | 200 | mg/day | Capsule, PO, Oral | Emtriva | emtricitabine | PDR |
| Max dose for adults | 240.0 | mg/day | 240 | mg/day | PO, oral | Emtriva | emtricitabine | PDR |
| Max dose for geriatric | 200.0 | mg/day | 200 | mg/day | Capsule, PO, Oral | Emtriva | emtricitabine | PDR |
| Max dose for geriatric | 240.0 | mg/day | 240 | mg/day | PO, oral | Emtriva | emtricitabine | PDR |