Basic Information
| Drug ID | DDPD00857 |

|
| Drug Name | Terbinafine | |
| Molecular Weight | 291.4299 | |
| Molecular Formula | C21H25N | |
| CAS Number | 91161-71-6 | |
| SMILES | CN(C\C=C\C#CC(C)(C)C)CC1=CC=CC2=CC=CC=C12 | |
| External Links | ||
| DRUGBANK | DB00857 | |
| PubChem Compound | 1549008 | |
| PDR | 2034 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
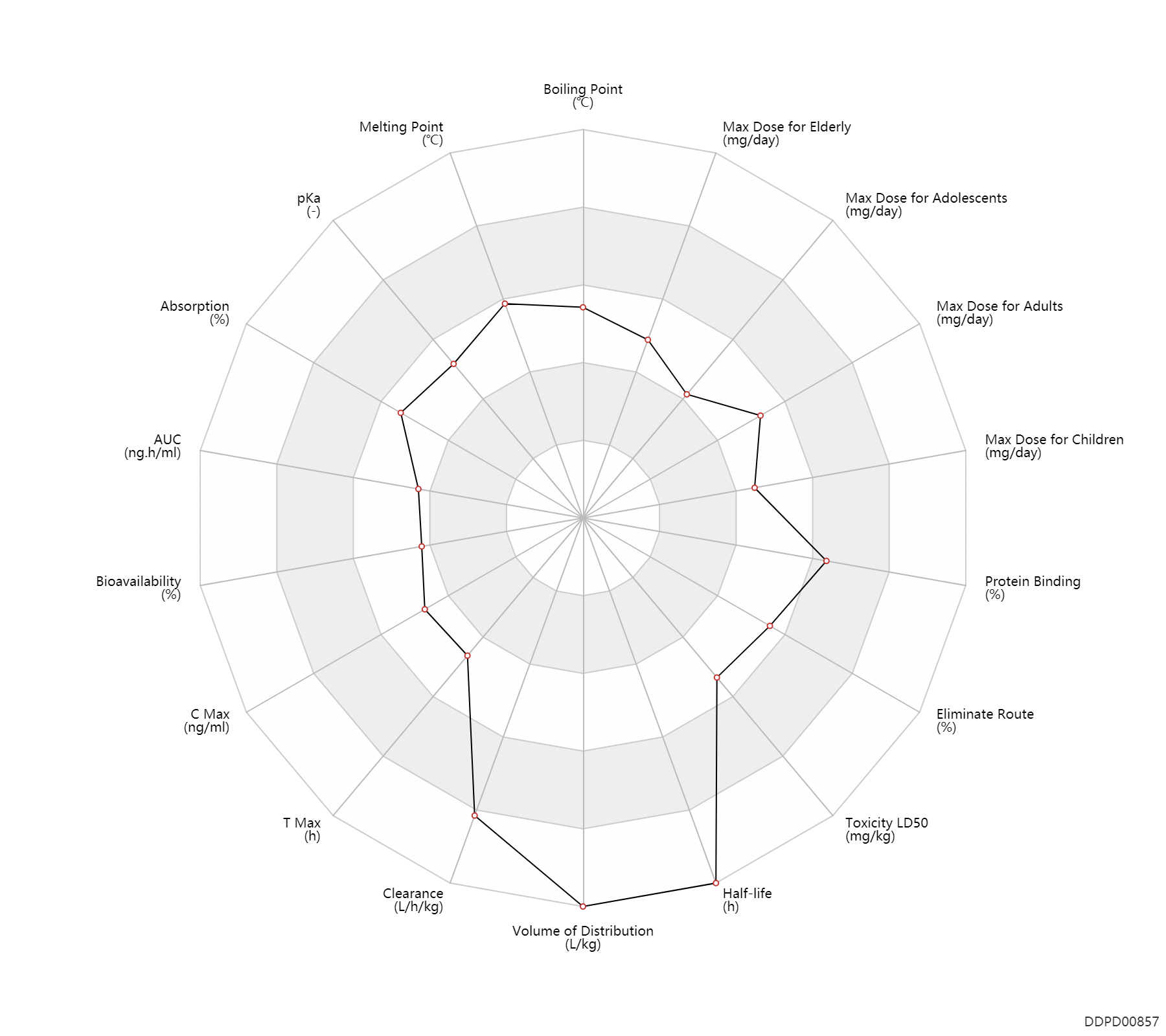
| Boiling Point | 417.9 | ℃ | 417.9 | ℃ | ChemSpider |
| Melting Point | 205.0 | ℃ | 205 | ℃ | Health Canada |
| Water Solubility | 0.63 | % | 0.63 | % | Health Canada |
| pKa | 7.1 | - | 7.1 | - | Health Canada |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 70.0 | % | 70 | % | DRUGBANK | |
| AUC | 4560.0 | ng.h/ml | 4.56 | ug.h/ml | DRUGBANK | AUC | 11593.0 | ng/cm2/h | 9694-13492 | ng/cm2/h | skin/dermal; | DRUGBANK |
| Bioavailability | 40.0 | % | 40 | % | DRUGBANK | |
| C Max | 1000.0 | ng/ml | 1.0 | ug/ml | DRUGBANK | C Max | 999.0 | ng/cm2 | 949-1049 | ng/cm2 | skin/dermal; | DRUGBANK |
| T Max | 2.0 | h | 2 | h | DRUGBANK | |
| Clearance | 1.1 | L/h/kg | 1.11 | L/h/kg | Oral single dose; | DRUGBANK |
| Volume of Distribution | 16.6 | L/kg | 16.6 | L/kg | at steady state; PO, oral; | DRUGBANK |
| Half-life | 36.0 | h | ~36 | h | effective half-life; PO, oral; | DRUGBANK | Half-life | 300.0 | h | 200-400 | h | terminal half-life; | DRUGBANK |
| Toxicity LD50 | 2000.0 | mg/kg | >2 | g/kg | subcutaneous injection, SC; Rattus, Rat; mouse; | DRUGBANK |
| Eliminate Route | 80.0 | % | ~80 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 20.0 | % | ~20 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 99.0 | % | >99 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 250.0 | mg/day | 250 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for children | 187.5 | mg/day | 187.5 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for children | 125.0 | mg/day | 125 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for adolescents | 250.0 | mg/day | 250 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for adolescents | 187.5 | mg/day | 187.5 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for adolescents | 125.0 | mg/day | 125 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for adults | 250.0 | mg/day | 250 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for adults | 500.0 | mg/day | 500 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for elderly | 250.0 | mg/day | 250 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |
| Max dose for elderly | 500.0 | mg/day | 500 | mg/day | PO, oral | Lamisil Granules | terbinafine hydrochloride | PDR |