Basic Information
| Drug ID | DDPD00847 |

|
| Drug Name | Cysteamine | |
| Molecular Weight | 77.149 | |
| Molecular Formula | C2H7NS | |
| CAS Number | 60-23-1 | |
| SMILES | NCCS | |
| External Links | ||
| DRUGBANK | DB00847 | |
| PubChem Compound | 6058 | |
| PDR | 1955 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
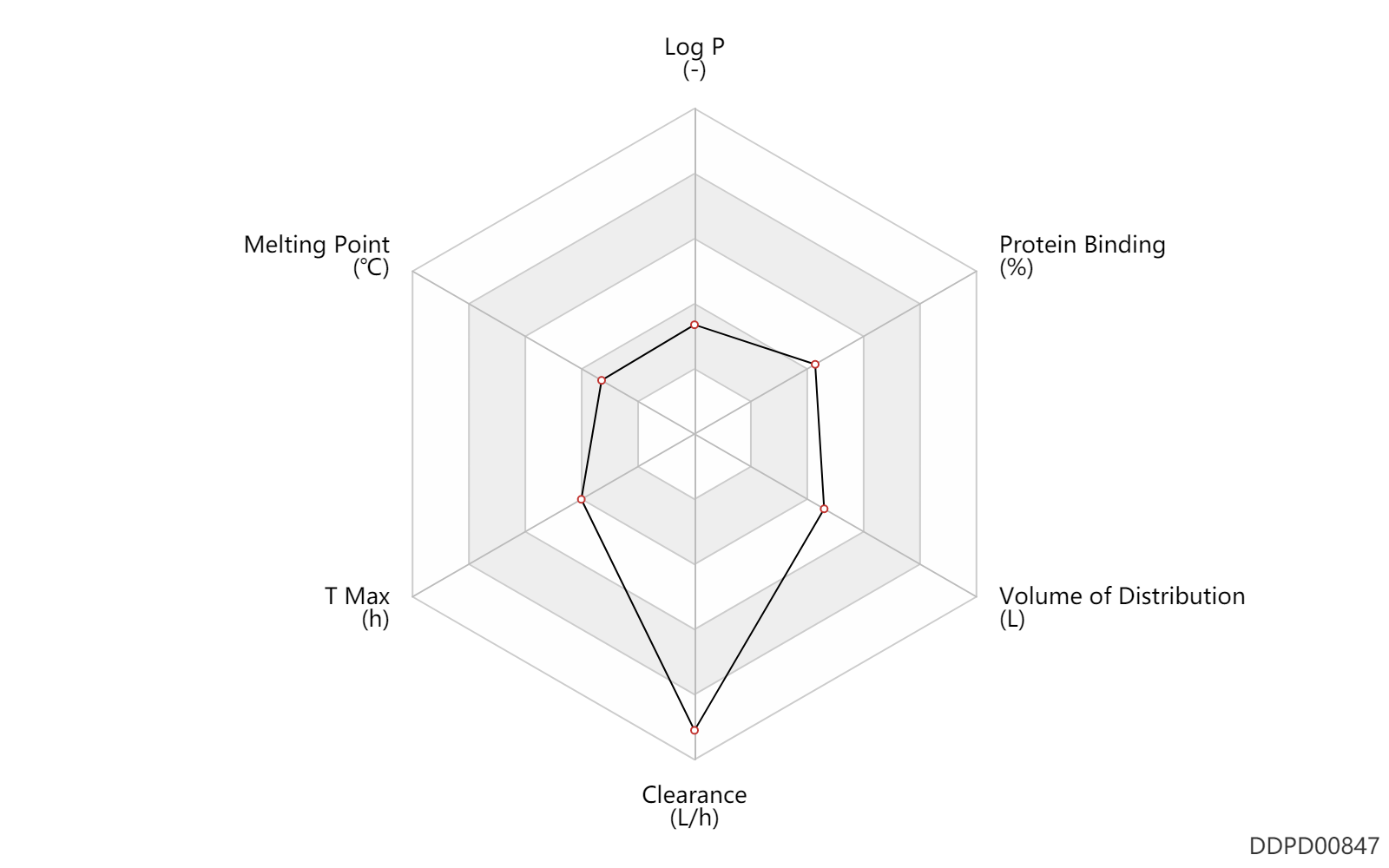
| Log P | 0.1 | - | 0.1 | - | DRUGBANK |
| Melting Point | 98.0 | ℃ | 98 | ℃ | PhysProp |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| T Max | 1.4 | h | 1.4 | h | DRUGBANK | |
| Clearance | 72.0 | L/h | ~1.2 | L/min | Plasma clearance; | DRUGBANK |
| Volume of Distribution | 156.0 | L | 156.0 | L | DRUGBANK | |
| Protein Binding | 52.0 | % | 52 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 1950.0 | mg/m2/day | 1.95 | g/m2/day | PO, oral | Cystagon | cysteamine bitartrate | PDR |
| Max dose for infants | 1.0 | drop/eye/h | 1 | drop/eye/h | ophthalmic administration; during waking hours | Cystagon | cysteamine bitartrate | PDR |
| Max dose for children | 1950.0 | mg/m2/day | 1.95 | g/m2/day | PO, oral | Cystagon | cysteamine bitartrate | PDR |
| Max dose for children | 1.0 | drop/eye/hour | 1 | drop/eye/hour | ophthalmic administration | Cystagon | cysteamine bitartrate | PDR |
| Max dose for adolescents | 1950.0 | mg/m2/day | 1.95 | g/m2/day | PO, oral | Cystagon | cysteamine bitartrate | PDR |
| Max dose for adolescents | 1.0 | drop/hour | 1 | drop/eye/hour | ophthalmic administration | Cystagon | cysteamine bitartrate | PDR |
| Max dose for adults | 1950.0 | mg/m2/day | 1.95 | g/m2/day | PO, oral | Cystagon | cysteamine bitartrate | PDR |
| Max dose for adults | 1.0 | drop/eye/h | 1 | drop/eye/h | ophthalmic administration | Cystagon | cysteamine bitartrate | PDR |
| Max dose for geriatric | 1.95 | g/m2/day | 1.95 | g/m2/day | PO, oral | Cystagon | cysteamine bitartrate | PDR |
| Max dose for geriatric | 1.0 | drop/eye/hour | 1 | drop/eye/hour | ophthalmic administration | Cystagon | cysteamine bitartrate | PDR |