Basic Information
| Drug ID | DDPD00839 |

|
| Drug Name | Tolazamide | |
| Molecular Weight | 311.4 | |
| Molecular Formula | C14H21N3O3S | |
| CAS Number | 1156-19-0 | |
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)NC(=O)NN1CCCCCC1 | |
| External Links | ||
| DRUGBANK | DB00839 | |
| T3DB | T3D4765 | |
| PubChem Compound | 5503 | |
| PDR | 1799 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
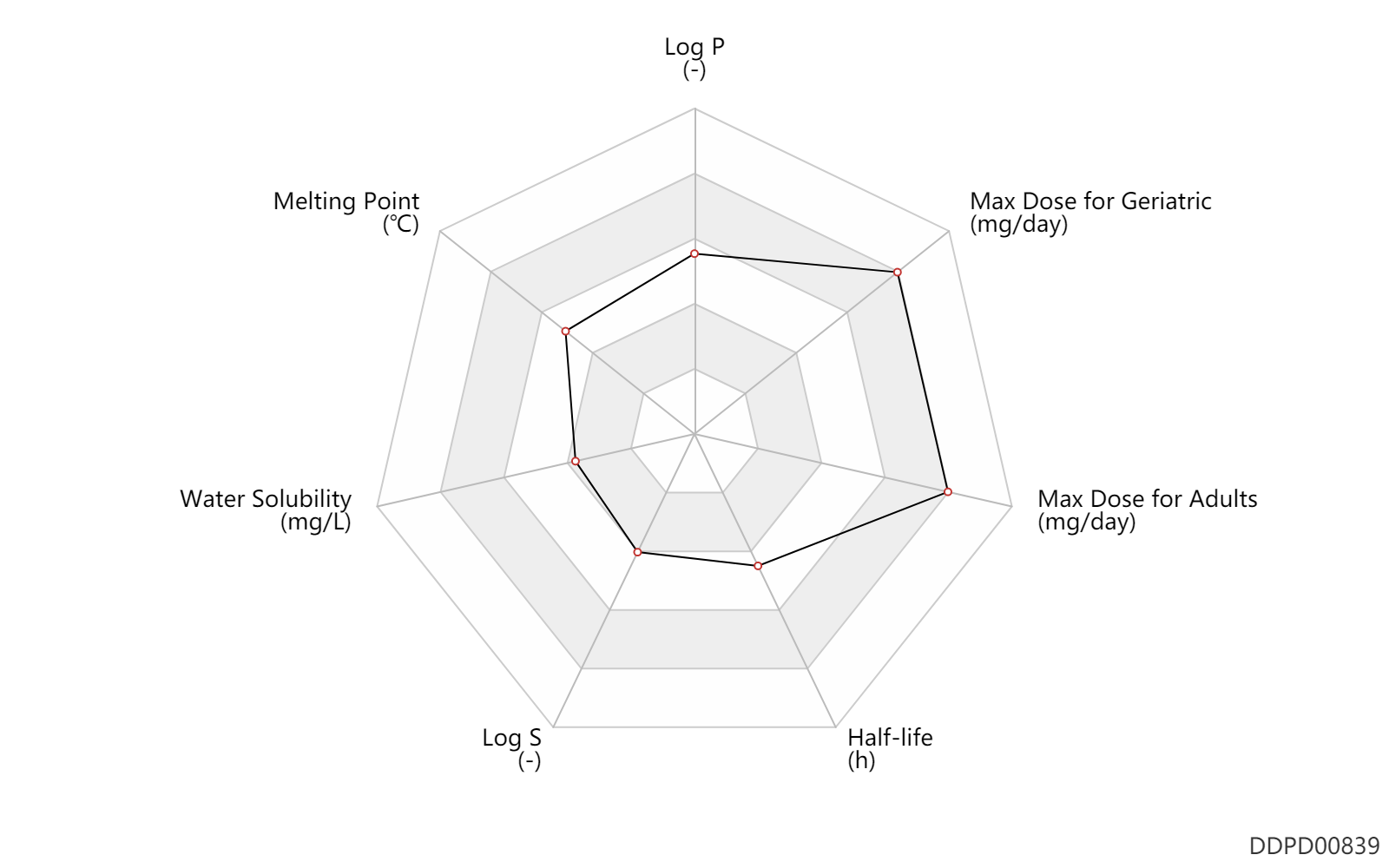
| Log P | 2.69 | - | 2.69 | - | SANGSTER (1993) |
| Melting Point | 171.5 | ℃ | 170-173 | ℃ | PhysProp |
| Water Solubility | 65.4 | mg/L | 65.4 | mg/L | YALKOWSKY,SH & DANNENFELSER,RM 1992) |
| Log S | -3.68 | - | -3.68 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Half-life | 7.0 | h | 7 | h | elimination half-life; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 1000.0 | mg/day | 1000 | mg/day | PO, oral | Tolazamide | tolazamide | PDR | |
| Max dose for geriatric | 1000.0 | mg/day | 1000 | mg/day | PO, oral | qd | Tolazamide | tolazamide | PDR |