Basic Information
| Drug ID | DDPD00834 |

|
| Drug Name | Mifepristone | |
| Molecular Weight | 429.5937 | |
| Molecular Formula | C29H35NO2 | |
| CAS Number | 84371-65-3 | |
| SMILES | [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@H](C1=CC=C(C=C1)N(C)C)C1=C3CCC(=O)C=C3CC[C@@]21[H] | |
| External Links | ||
| DRUGBANK | DB00834 | |
| T3DB | T3D2907 | |
| PubChem Compound | 55245 | |
| PDR | 2928 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
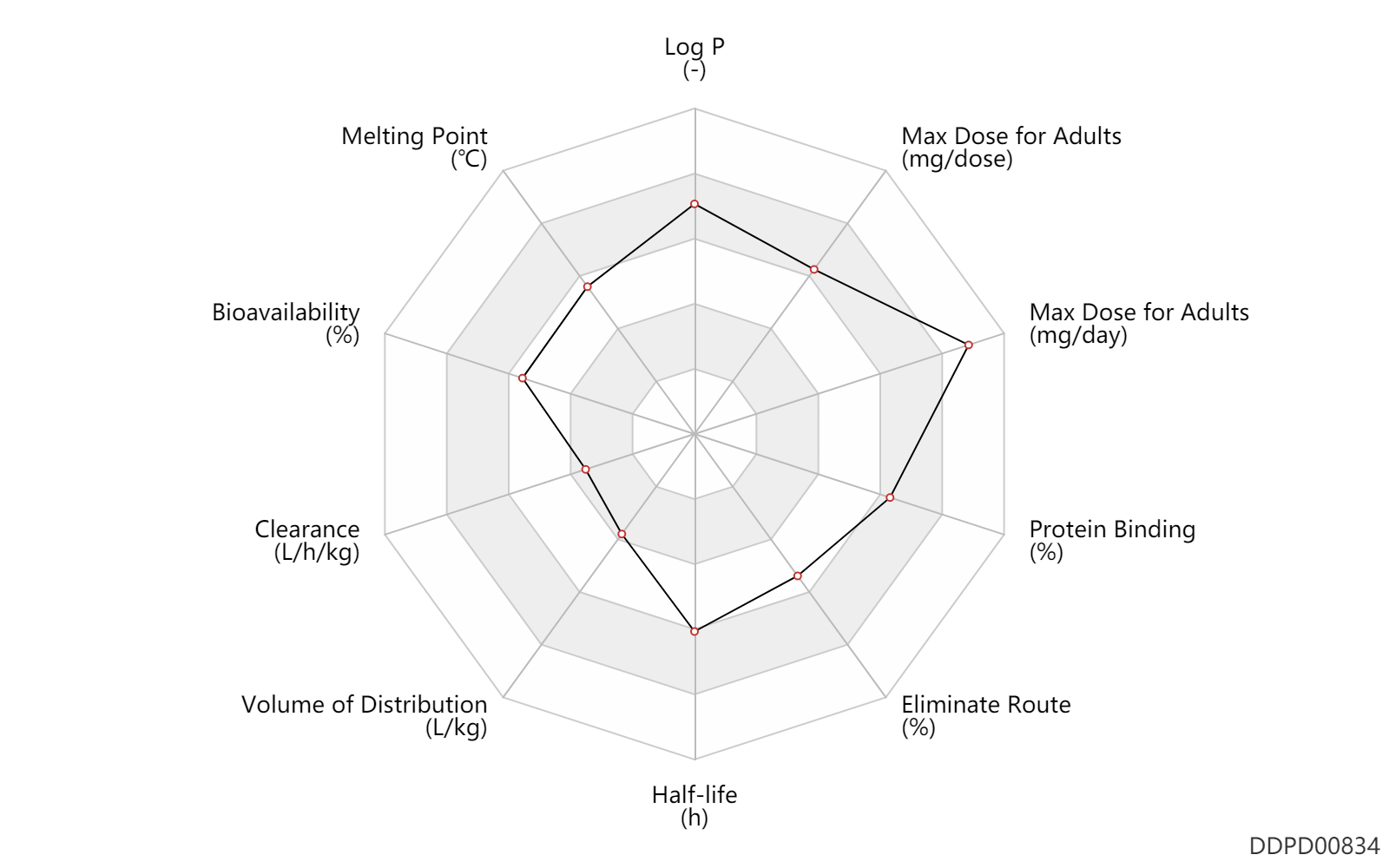
| Log P | 4.5 | - | 4.5 | - | DRUGBANK |
| Melting Point | 193.5 | ℃ | 191-196 | ℃ | FDA Label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 69.0 | % | 69 | % | PO, oral; | DRUGBANK |
| Clearance | 0.0300 | L/h/kg | 0.5 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.45 | L/kg | 0.45 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 18.0 | h | 18 | h | DRUGBANK | Half-life | 16.6 | h | 16.6 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 83.0 | % | 83 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 9.0 | % | 9 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 98.0 | % | 98 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 200.0 | mg/dose | 200 | mg/dose | Korlym | mifepristone | PDR | |
| Max dose for adults | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Korlym | mifepristone | PDR |
| Max dose for adults | 20.0 | mg/kg/day | 20 | mg/kg/day | PO, oral | Korlym | mifepristone | PDR |