Basic Information
| Drug ID | DDPD00808 |

|
| Drug Name | Indapamide | |
| Molecular Weight | 365.835 | |
| Molecular Formula | C16H16ClN3O3S | |
| CAS Number | 26807-65-8 | |
| SMILES | CC1CC2=CC=CC=C2N1NC(=O)C1=CC(=C(Cl)C=C1)S(N)(=O)=O | |
| External Links | ||
| DRUGBANK | DB00808 | |
| PubChem Compound | 3702 | |
| PDR | 2292 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
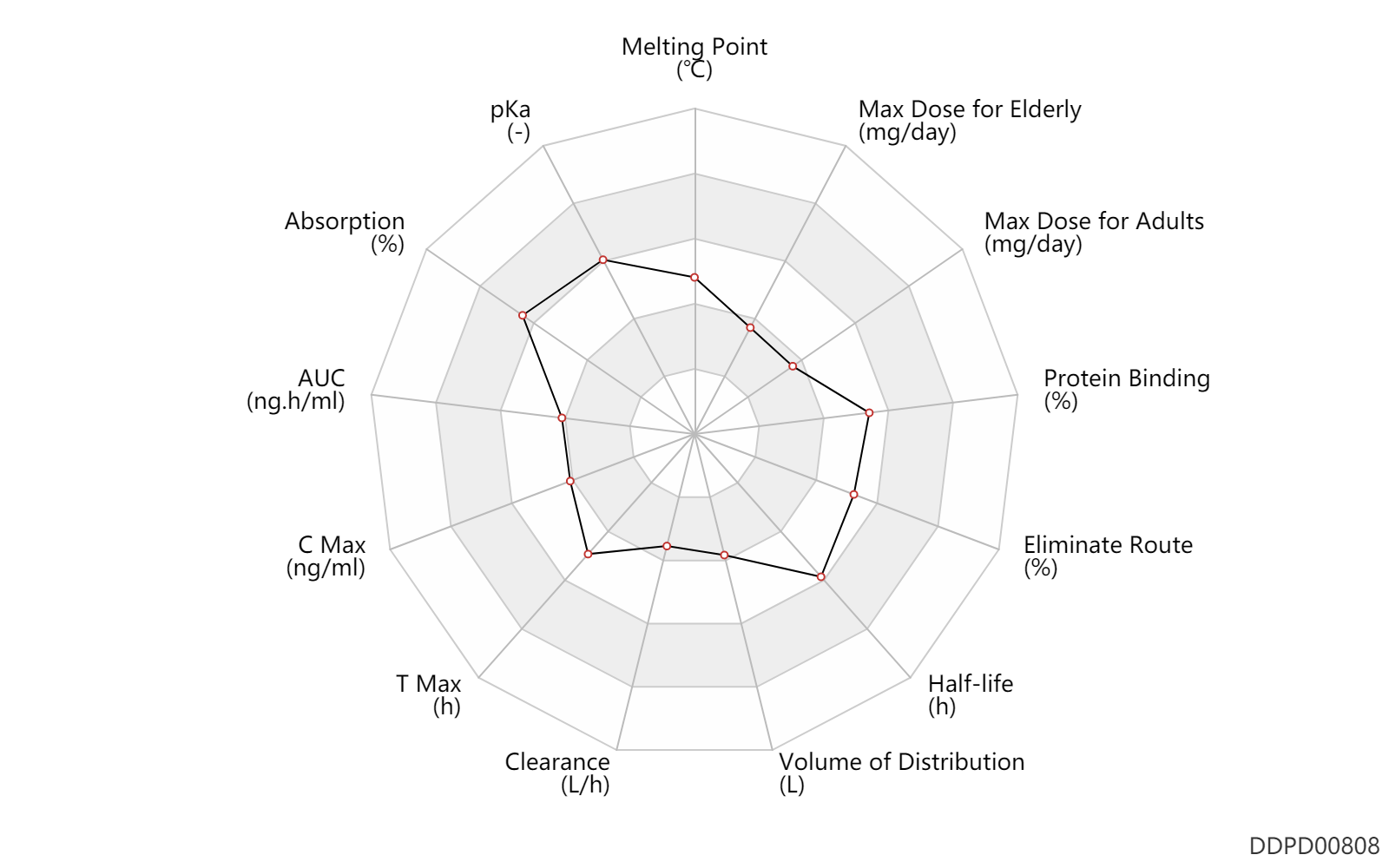
| Melting Point | 161.0 | ℃ | 160-162 | ℃ | https://www.fishersci.com/store/msds?partNumber=AAJ6384603&productDescription=INDAPAMIDE+1G&vendorId=VN00024248&countryCode=US&language=en |
| pKa | 8.8 | - | 8.8 | - | FDA Label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | 100 | % | PO, oral; food; | food → ; | DRUGBANK |
| AUC | 2950.0 | ng.h/ml | 2.95 | ug.h/ml | PO, oral; | DRUGBANK | |
| C Max | 363.0 | ng/ml | 363.0 | ng/ml | PO, oral; | DRUGBANK | |
| T Max | 2.3 | h | 2.3 | h | PO, oral; | DRUGBANK | |
| Clearance | 0.10 | L/h | 1.71 | ml/min | Renal clearance; | DRUGBANK | Clearance | 1.3 | L/h | 20-23.4 | ml/min | Total clearance; | DRUGBANK |
| Volume of Distribution | 42.5 | L | 25-60 | L | Apparent volume of distribution; | DRUGBANK | |
| Half-life | 16.0 | h | 13.9-18 | h | elimination half-life; normal,healthy; | DRUGBANK | |
| Eliminate Route | 65.0 | % | 60-70 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 19.5 | % | 16-23 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 77.5 | % | ~76-79 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 5.0 | mg/day | 5 | mg/day | PO, oral | Indapamide | indapamide | PDR |
| Max dose for elderly | 5.0 | mg/day | 5 | mg/day | PO, oral | Indapamide | indapamide | PDR |