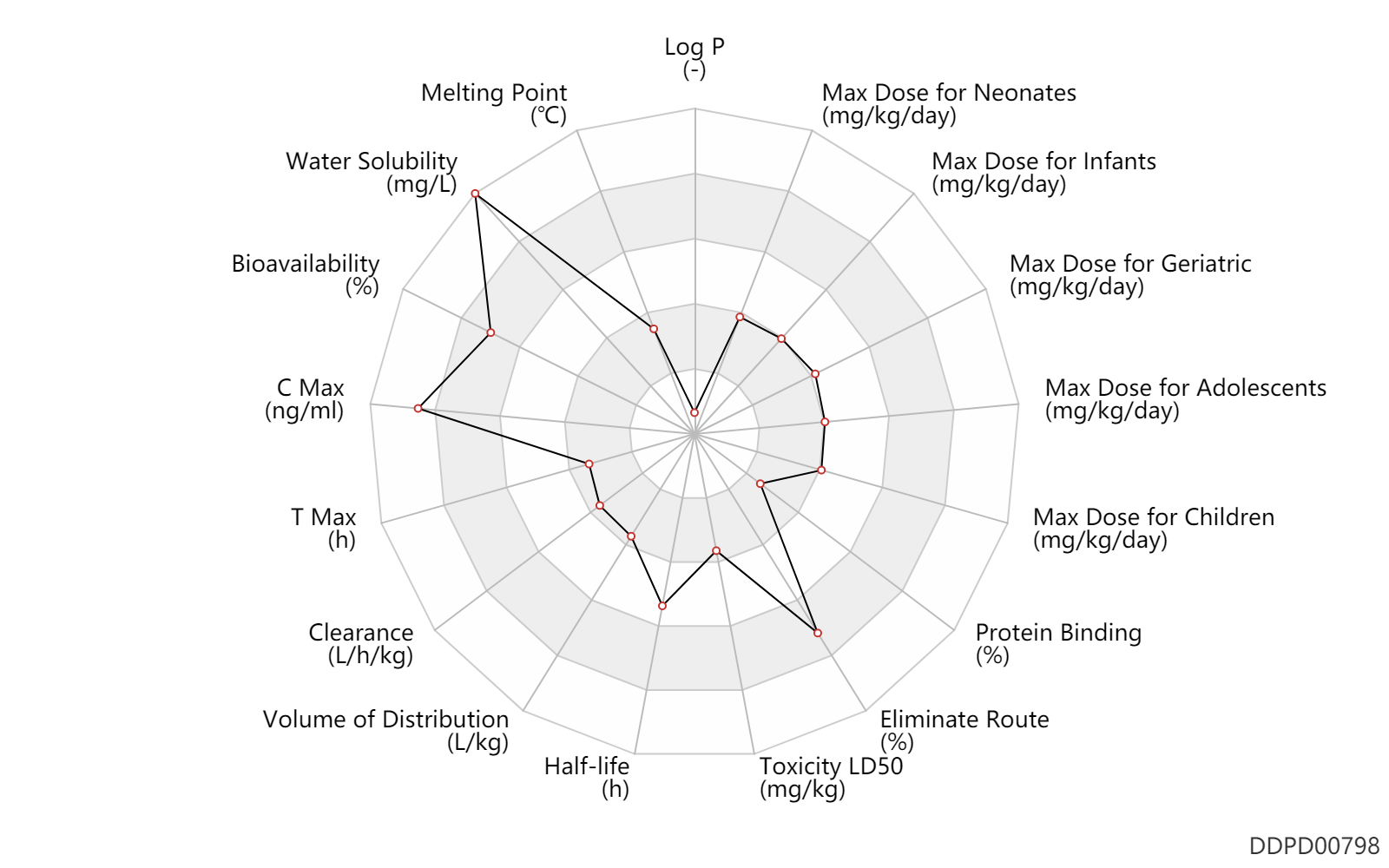
| Max dose for neonates |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for neonates |
5.0 |
mg/kg/day |
5 |
mg/kg/dose |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for neonates |
10.0 |
mg/kg/day |
5 |
mg/kg/dose |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for neonates |
4.5 |
mg/kg/day |
4.5 |
mg/kg/dose/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for infants |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for children |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for adolescents |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for adults |
5.0 |
mg/kg/day |
5 |
mg/kg/day |
PO, oral;intravenous injection, IV; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for adults |
7.0 |
mg/kg/day |
7 |
mg/kg/day |
intravenous injection, IV |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for geriatric |
5.0 |
mg/kg/day |
5 |
mg/kg/day |
PO, oral;intravenous injection, IV; |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for geriatric |
7.0 |
mg/kg/day |
7 |
mg/kg/day |
intravenous injection, IV |
Gentamicin Sulfate Ointment |
gentamicin sulfate |
PDR |
| Max dose for neonates |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for neonates |
5.0 |
mg/kg/day |
5 |
mg/kg/dose |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for neonates |
10.0 |
mg/kg/day |
5 |
mg/kg/dose |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for neonates |
4.5 |
mg/kg/day |
4.5 |
mg/kg/dose/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for infants |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for children |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for adolescents |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for adults |
5.0 |
mg/kg/day |
5 |
mg/kg/day |
PO, oral;intravenous injection, IV; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for adults |
7.0 |
mg/kg/day |
7 |
mg/kg/day |
intravenous injection, IV |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for geriatric |
5.0 |
mg/kg/day |
5 |
mg/kg/day |
PO, oral;intravenous injection, IV; |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for geriatric |
7.0 |
mg/kg/day |
7 |
mg/kg/day |
intravenous injection, IV |
Gentamicin Injection 40 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for neonates |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for neonates |
5.0 |
mg/kg/day |
5 |
mg/kg/dose |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for neonates |
10.0 |
mg/kg/day |
5 |
mg/kg/dose |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for neonates |
4.5 |
mg/kg/day |
4.5 |
mg/kg/dose/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for infants |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for children |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for adolescents |
7.5 |
mg/kg/day |
7.5 |
mg/kg/day |
intravenous injection, IV;IM,intramuscular injection; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for adults |
5.0 |
mg/kg/day |
5 |
mg/kg/day |
PO, oral;intravenous injection, IV; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for adults |
7.0 |
mg/kg/day |
7 |
mg/kg/day |
intravenous injection, IV |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for geriatric |
5.0 |
mg/kg/day |
5 |
mg/kg/day |
PO, oral;intravenous injection, IV; |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |
| Max dose for geriatric |
7.0 |
mg/kg/day |
7 |
mg/kg/day |
intravenous injection, IV |
Gentamicin Injection 10 mg/mL |
gentamicin sulfate |
PDR |