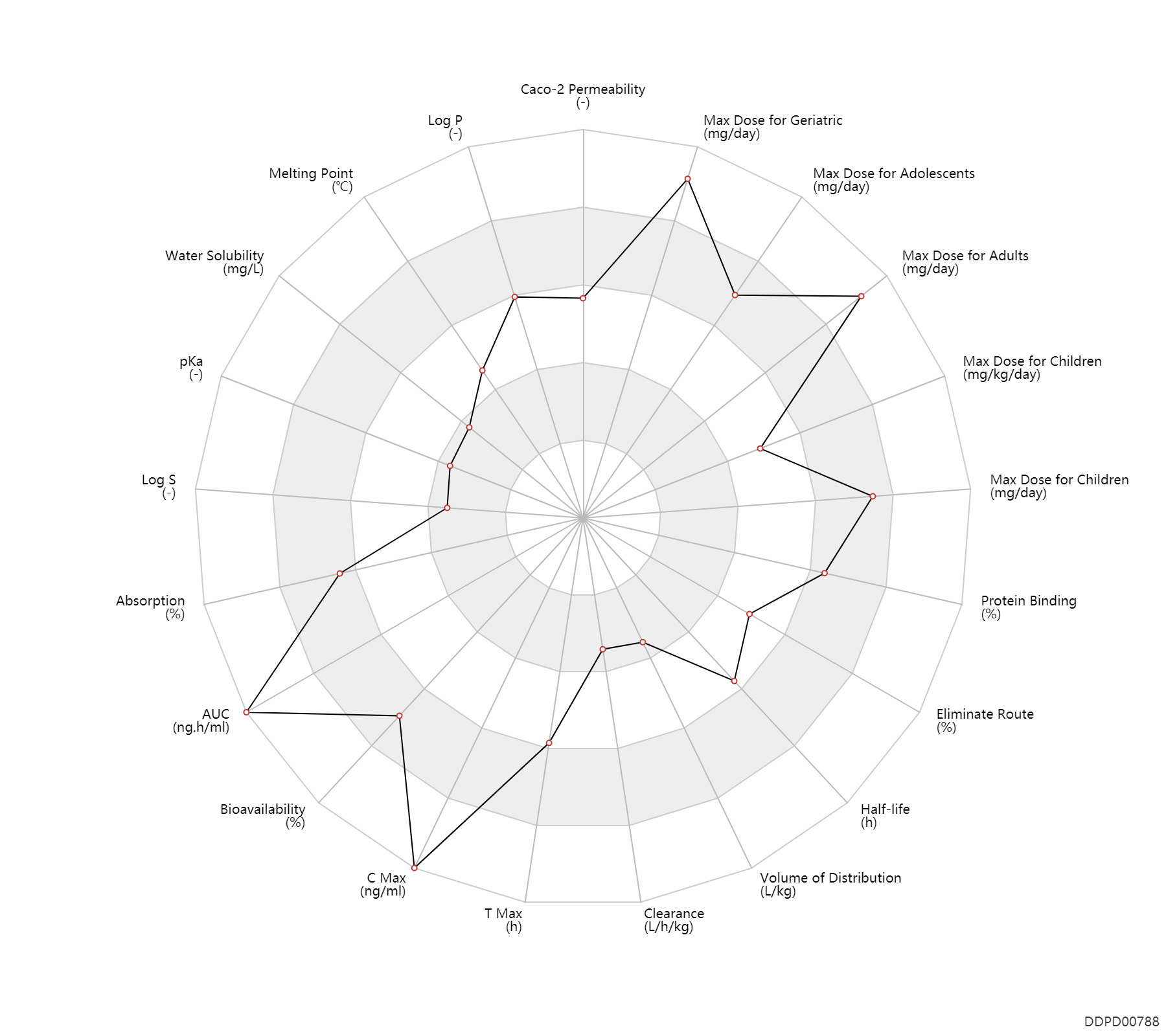
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Factor |
Reference |
| Absorption |
100.0 |
% |
100 |
% |
PO, oral; Rectal Administration; |
|
DRUGBANK |
| AUC |
1446000.0 |
ng.h/ml |
1446.0 |
mcg.h/ml |
PO, oral; Rectal Administration; immediate release formulation; |
|
DRUGBANK |
AUC |
1448000.0 |
ng.h/ml |
1448.0 |
mcg.h/ml |
PO, oral; Rectal Administration; extended release formulation; |
|
DRUGBANK |
AUC |
845000.0 |
ng.h/ml |
845.0 |
mcg.h/ml |
PO, oral; Derivative; |
|
DRUGBANK |
AUC |
767000.0 |
ng.h/ml |
767.0 |
mcg.h/ml |
PO, oral; Rectal Administration; different study; |
|
DRUGBANK |
| Bioavailability |
99.0 |
% |
99.0 |
% |
|
|
The Pharmacological Basis of Therapeutics |
| C Max |
95700.0 |
ng/ml |
94-97.4 |
mcg/ml |
PO, oral; Rectal Administration; |
|
DRUGBANK |
C Max |
37.0 |
null |
37.0 |
null |
Tablet, PO, oral; immediate release formulation; adults; |
|
The Pharmacological Basis of Therapeutics |
C Max |
94.0 |
null |
94.0 |
null |
Tablet, PO, oral; extended release formulation; adults; |
|
The Pharmacological Basis of Therapeutics |
C Max |
55000.0 |
ng/ml |
55±14 |
mcg/ml |
Liquid; Children; patients; |
|
The Pharmacological Basis of Therapeutics |
| T Max |
2.0 |
h |
2 |
h |
PO, oral; Rectal Administration; |
|
DRUGBANK |
T Max |
3.0 |
h |
3 |
h |
PO, oral; Rectal Administration; |
|
DRUGBANK |
T Max |
5.0 |
h |
5 |
h |
PO, oral; Rectal Administration; |
|
DRUGBANK |
T Max |
4.0 |
h |
4 |
h |
PO, oral; Derivative; |
|
DRUGBANK |
T Max |
1.9 |
h |
1.9 |
h |
PO, oral; Rectal Administration; different study; |
|
DRUGBANK |
T Max |
5.0 |
h |
5 |
h |
combination drug use; |
|
DRUGBANK |
T Max |
1.0 |
h |
1 |
h |
PO, oral; Derivative; |
|
DRUGBANK |
T Max |
3.0 |
h |
2-4 |
h |
Tablet, PO, oral; immediate release formulation; adults; |
|
The Pharmacological Basis of Therapeutics |
T Max |
5.0 |
h |
5.0 |
h |
Tablet, PO, oral; extended release formulation; adults; |
|
The Pharmacological Basis of Therapeutics |
T Max |
2.2 |
h |
2.2±2.1 |
h |
Liquid; Children; patients; |
|
The Pharmacological Basis of Therapeutics |
| Clearance |
0.007800 |
L/h/kg |
0.13 |
ml/min/kg |
|
|
DRUGBANK |
Clearance |
0.007800 |
L/h/kg |
0.13±0.02 |
ml/min/kg |
apparent clearance; hydrolysis; hydrolysis; |
Children → ;Elderly → ;Hepatic cirrhosis, cirr → ;RD, renal impairment, Renal disease,including uremia ↓ ;rheumatoid arthritis ↑ ; |
The Pharmacological Basis of Therapeutics |
Clearance |
0.004200 |
L/h/kg |
0.07 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
0.16 |
L/kg |
0.16 |
L/kg |
|
|
DRUGBANK |
Volume of Distribution |
0.16 |
L/kg |
0.16±0.02 |
L/kg |
Apparent volume of distribution; hydrolysis; hydrolysis; |
Elderly → ;RD, renal impairment, Renal disease,including uremia ↑ ;rheumatoid arthritis ↑ ; |
The Pharmacological Basis of Therapeutics |
Volume of Distribution |
0.0900 |
L/kg |
0.09 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
14.5 |
h |
12-17 |
h |
elimination half-life; |
|
DRUGBANK |
Half-life |
14.0 |
h |
14±1 |
h |
|
Children → ;RD, renal impairment, Renal disease,including uremia → ;rheumatoid arthritis → ;Age ↑ ; |
The Pharmacological Basis of Therapeutics |
Half-life |
16.6 |
h |
16.63 |
h |
intravenous injection, IV; human, homo sapiens; |
|
Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route |
95.0 |
% |
~95 |
% |
Urinary excretion; PO, oral; |
|
DRUGBANK |
Eliminate Route |
5.0 |
% |
<5 |
% |
Faeces excretion; PO, oral; |
|
DRUGBANK |
Eliminate Route |
5.5 |
% |
5-6 |
% |
Urinary excretion; Unchanged drug; |
|
The Pharmacological Basis of Therapeutics |
| Protein Binding |
99.0 |
% |
>99 |
% |
|
|
DRUGBANK |
Protein Binding |
99.7 |
% |
99.7±0.1 |
% |
|
Elderly ↑ ;RD, renal impairment, Renal disease,including uremia ↑ ;Hepatic cirrhosis, cirr ↑ ;rheumatoid arthritis ↓ ;hypoalbuminemia Alb ↓ ; |
The Pharmacological Basis of Therapeutics |