Basic Information
| Drug ID | DDPD00787 |

|
| Drug Name | Acyclovir | |
| Molecular Weight | 225.2046 | |
| Molecular Formula | C8H11N5O3 | |
| CAS Number | 59277-89-3 | |
| SMILES | NC1=NC(=O)C2=C(N1)N(COCCO)C=N2 | |
| External Links | ||
| DRUGBANK | DB00787 | |
| PubChem Compound | 2022 | |
| PDR | 1341 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
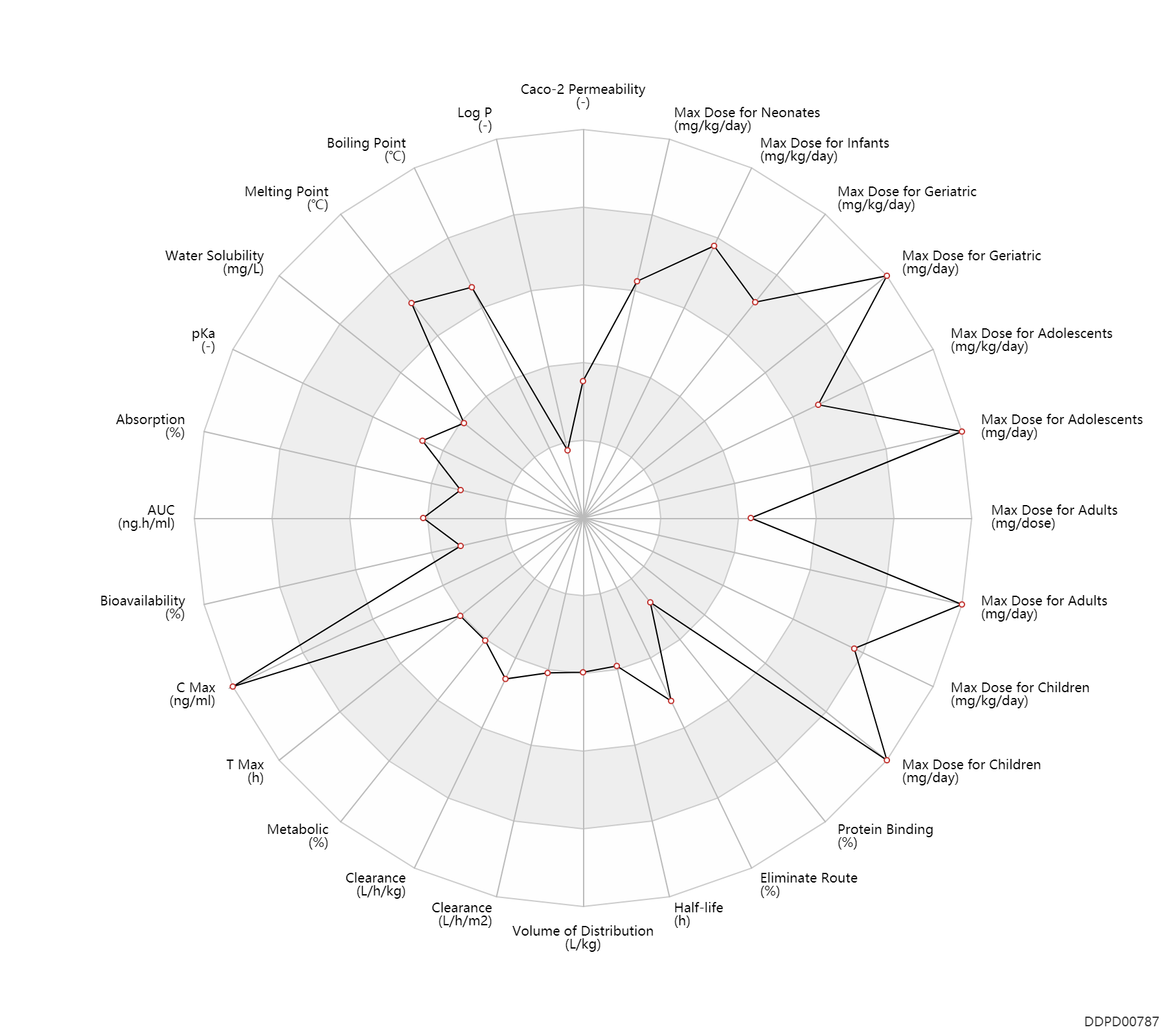
| Caco-2 Permeability | -6.15 | - | -6.15 | - | ADME Research, USCD |
| Log P | -1.76 | - | -1.76 | - | http://www.chemspider.com/Chemical-Structure.1945.html |
| Boiling Point | 595.0 | ℃ | 595 | ℃ | http://www.chemspider.com/Chemical-Structure.1945.html |
| Melting Point | 255.0 | ℃ | 255 | ℃ | http://www.chemspider.com/Chemical-Structure.1945.html |
| Water Solubility | 1410.0 | mg/L | 1.41 | mg/ml | Avaclyr Ophthalmic Ointment FDA label |
| pKa | 2.52 | - | 2.52,9.35 | - | Avaclyr Ophthalmic Ointment FDA label | pKa | 9.35 | - | 2.52,9.35 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Absorption | 4.7 | % | <0.02-9.4 | % | skin/dermal; | DRUGBANK | |
| AUC | 3029.6 | ng.h/ml | 2956.6-3102.5 | ng.h/ml | DRUGBANK | ||
| Bioavailability | 15.0 | % | 10-20 | % | PO, oral; increasing doses; food; | increasing doses ↑ ;food → ; | DRUGBANK | Bioavailability | 22.5 | % | 15-30 | % | PO, oral; | increasing doses ↓ ; | The Pharmacological Basis of Therapeutics |
| C Max | 625.1 | ng/ml | 593.7-656.5 | ng/ml | DRUGBANK | C Max | 1002160.5 | ng/ml | 3.5-5.4 | mM | Oral multiple dose; | The Pharmacological Basis of Therapeutics |
| T Max | 1.1 | h | 1.1±0.4 | h | DRUGBANK | T Max | 1.8 | h | 1.5-2 | h | Oral multiple dose; | The Pharmacological Basis of Therapeutics |
| Metabolic | 15.0 | % | <15 | % | DRUGBANK | Metabolic | 1.0 | % | 1 | % | DRUGBANK |
| Clearance | 8.6 | L/h/m2 | 248.0 | ml/min/1.73m2 | Renal clearance; | DRUGBANK | Clearance | 4.2 | L/h/m2 | 122.0 | ml/min/1.73m2 | Total clearance; Neonates; | DRUGBANK | Clearance | 0.28 | L/h/kg | 4.7 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.60 | L/kg | 0.6 | L/kg | DRUGBANK | Volume of Distribution | 0.69 | L/kg | 0.69±0.19 | L/kg | Neonates ↓ ;RD, renal impairment, Renal disease,including uremia → ; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 0.71 | L/kg | 0.71 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 5.0 | h | ~5 | h | elimination half-life; | DRUGBANK | Half-life | 2.6 | h | 2.6 | h | Children; | DRUGBANK | Half-life | 2.4 | h | 2.4±0.7 | h | Neonates ↑ ;Children → ;RD, renal impairment, Renal disease,including uremia ↑ ; | The Pharmacological Basis of Therapeutics | Half-life | 2.5 | h | 2.5 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 0.10 | % | <0.1 | % | lung excretion; | DRUGBANK | Eliminate Route | 2.0 | % | <2 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 91.0 | % | 90-92 | % | Unchanged drug; | DRUGBANK | Eliminate Route | 75.0 | % | 75±10 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 21.0 | % | 9-33 | % | plasma proteins; | DRUGBANK | Protein Binding | 15.0 | % | 15±4 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 60.0 | mg/kg/day | 60 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for neonates | 900.0 | mg/m2/day | 900 | mg/m2/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for infants | 60.0 | mg/kg/day | 60 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for infants | 80.0 | mg/kg/day | 80 | mg/kg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 30.0 | mg/kg/day | 30 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 45.0 | mg/kg/day | 45 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 80.0 | mg/kg/day | 80 | mg/kg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 3200.0 | mg/day | 3200 | mg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 4000.0 | mg/day | 4000 | mg/day | Zovirax Ointment | acyclovir | PDR | |
| Max dose for children | 60.0 | mg/kg/day | 60 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 80.0 | mg/kg/day | 80 | mg/kg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 3200.0 | mg/day | 3200 | mg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 60.0 | mg/kg/day | 60 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for children | 80.0 | mg/kg/day | 80 | mg/kg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for adolescents | 30.0 | mg/kg/day | 30 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for adolescents | 45.0 | mg/kg/day | 45 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for adolescents | 80.0 | mg/kg/day | 80 | mg/kg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for adolescents | 3200.0 | mg/day | 3200 | mg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for adolescents | 4000.0 | mg/day | 4000 | mg/day | Zovirax Ointment | acyclovir | PDR | |
| Max dose for adults | 30.0 | mg/kg/day | 30 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for adults | 45.0 | mg/kg/day | 45 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for adults | 4000.0 | mg/day | 4000 | mg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for adults | 50.0 | mg/dose | 50 | mg/dose | buccal tablets | Zovirax Ointment | acyclovir | PDR |
| Max dose for geriatric | 30.0 | mg/kg/day | 30 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for geriatric | 45.0 | mg/kg/day | 45 | mg/kg/day | intravenous injection, IV | Zovirax Ointment | acyclovir | PDR |
| Max dose for geriatric | 4000.0 | mg/day | 4000 | mg/day | PO, oral | Zovirax Ointment | acyclovir | PDR |
| Max dose for geriatric | 50.0 | mg/dose | 50 | mg/dose | buccal tablets | Zovirax Ointment | acyclovir | PDR |