Basic Information
| Drug ID | DDPD00775 |

|
| Drug Name | Tirofiban | |
| Molecular Weight | 440.597 | |
| Molecular Formula | C22H36N2O5S | |
| CAS Number | 144494-65-5 | |
| SMILES | CCCCS(=O)(=O)N[C@@H](CC1=CC=C(OCCCCC2CCNCC2)C=C1)C(O)=O | |
| External Links | ||
| DRUGBANK | DB00775 | |
| PubChem Compound | 60947 | |
| PDR | 662 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
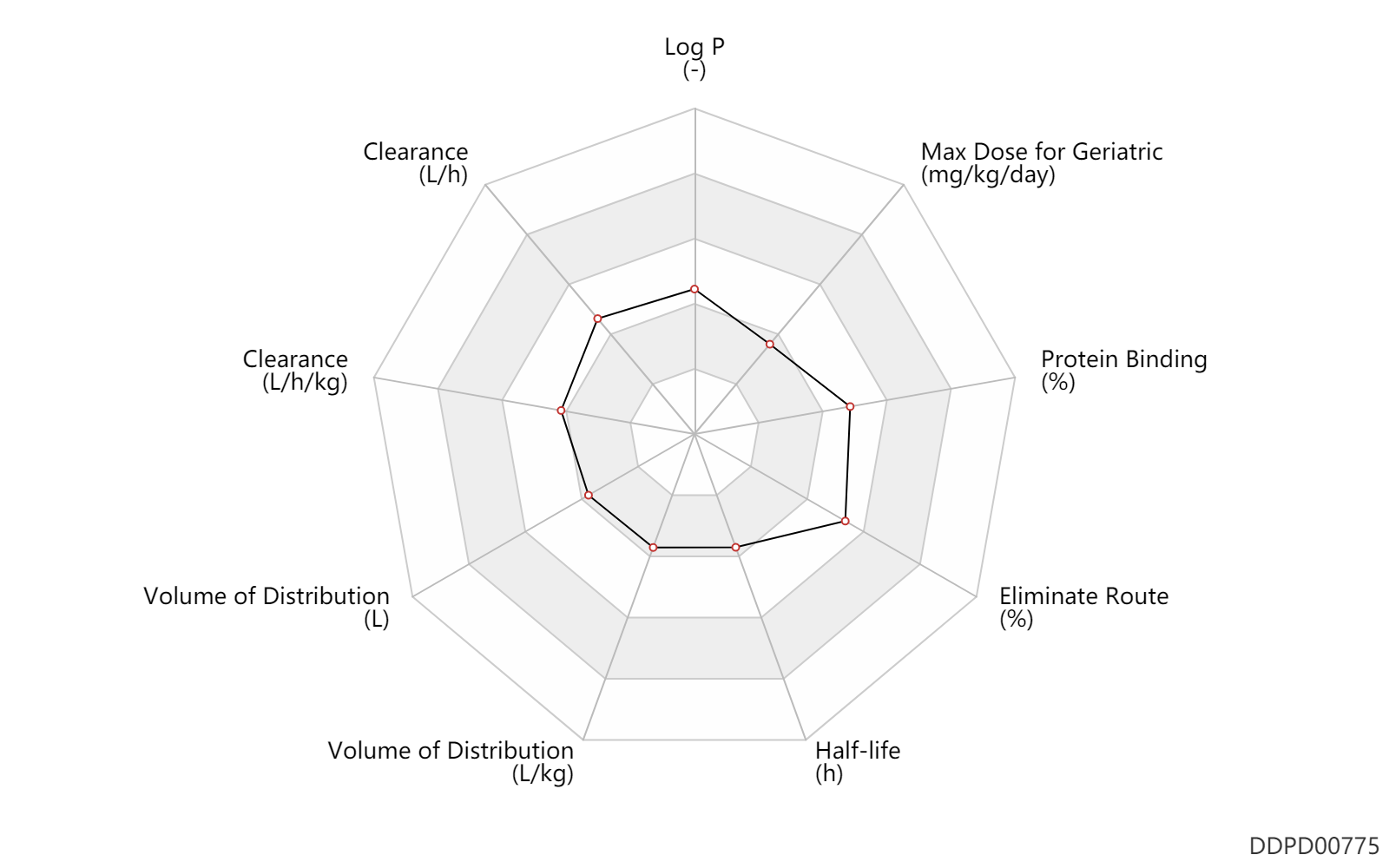
| Log P | 1.4 | - | 1.4 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Clearance | 16.6 | L/h | 213-314 | ml/min | normal,healthy; | DRUGBANK | Clearance | 12.6 | L/h | 152-267 | ml/min | coronary artery disease; patients; | DRUGBANK | Clearance | 0.18 | L/h/kg | 3 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 32.0 | L | 22-42 | L | DRUGBANK | Volume of Distribution | 0.34 | L/kg | 0.34 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 2.0 | h | 2 | h | DRUGBANK | Half-life | 1.6 | h | 1.6 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 65.0 | % | ~65 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 25.0 | % | ~25 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 65.0 | % | 65 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 0.025 | mg/kg | 25 | mcg/kg | intravenous injection, IV | Aggrastat | tirofiban hydrochloride | PDR |
| Max dose for adults | 0.216 | mg/kg/day | 0.15 | mcg/kg/min | intravenous infusion, iv in drop; up to 18 hours | Aggrastat | tirofiban hydrochloride | PDR |
| Max dose for geriatric | 25.0 | mcg/kg | 25 | mcg/kg | intravenous injection, IV | Aggrastat | tirofiban hydrochloride | PDR |
| Max dose for geriatric | 0.216 | mg/kg/day | 0.15 | mcg/kg/minute | intravenous infusion, iv in drop; up to 18 hours | Aggrastat | tirofiban hydrochloride | PDR |