Basic Information
| Drug ID | DDPD00759 |

|
| Drug Name | Tetracycline | |
| Molecular Weight | 444.4346 | |
| Molecular Formula | C22H24N2O8 | |
| CAS Number | 60-54-8 | |
| SMILES | [H][C@@]12C[C@@]3([H])C(=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@H]2N(C)C)C(=O)C1=C(O)C=CC=C1[C@@]3(C)O | |
| External Links | ||
| DRUGBANK | DB00759 | |
| T3DB | T3D3955 | |
| PubChem Compound | 54675776 | |
| PDR | 3315 | |
| Drugs.com | Drugs.com Drug Page | |
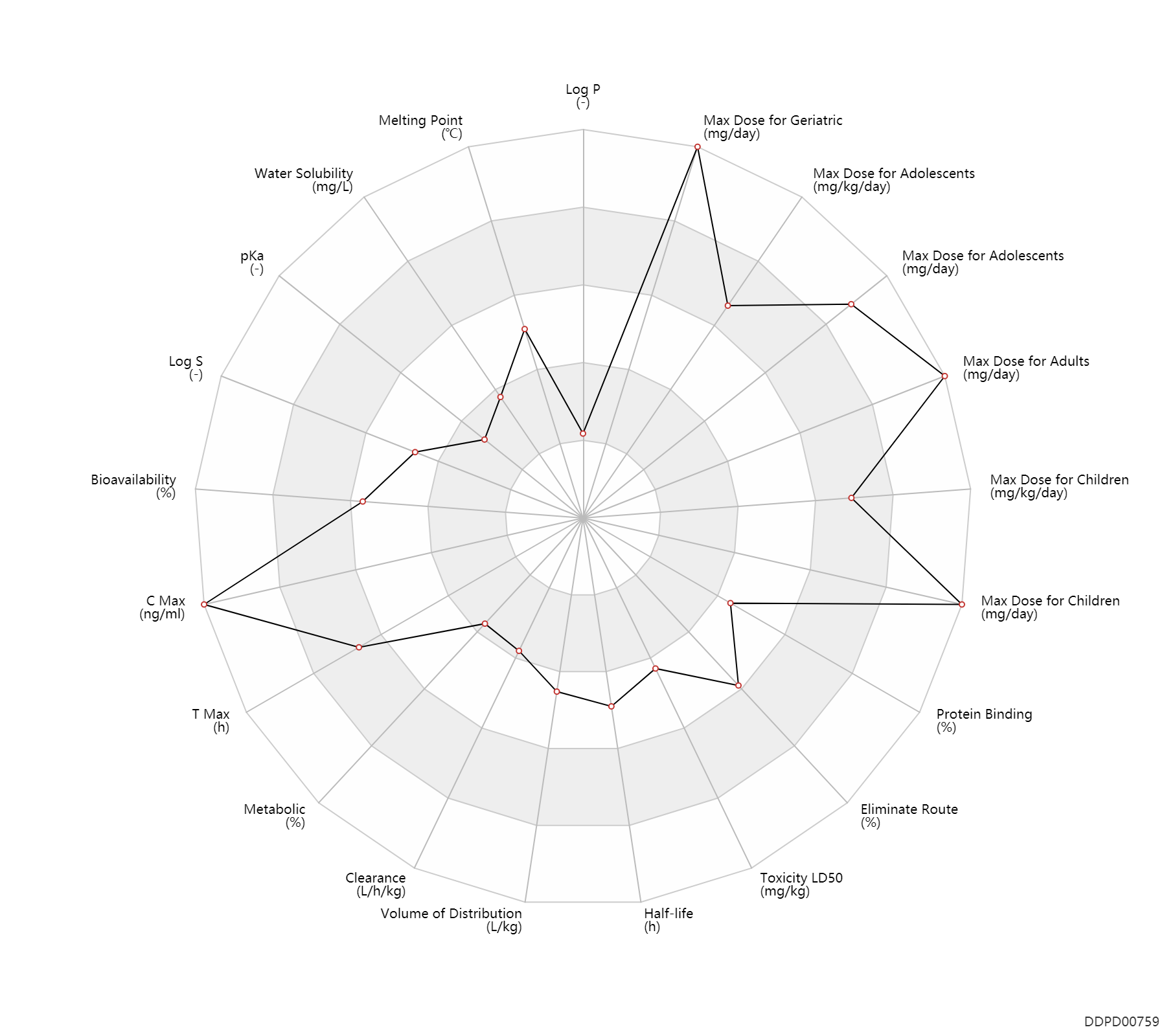
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | -1.3 | - | -1.3 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 172.5 | ℃ | 172.5 | ℃ | PhysProp |
| Water Solubility | 231.0 | mg/L | 231 | mg/L | YALKOWSKY,SH & DANNENFELSER,RM 1992) |
| pKa | 3.3 | - | 3.3 | - | SERJEANT,EP & DEMPSEY,B (1979) |
| Log S | -3.12 | - | -3.12 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 40.0 | % | <40 | % | IM,intramuscular injection; fasting; adults; | DRUGBANK | Bioavailability | 100.0 | % | 100 | % | intravenous injection, IV; fasting; adults; | DRUGBANK | Bioavailability | 70.0 | % | 60-80 | % | PO, oral; fasting; adults; | DRUGBANK | Bioavailability | 77.0 | % | 77.0 | % | PO, oral; | The Pharmacological Basis of Therapeutics |
| C Max | 2300.0 | ng/ml | 2.3±0.2 | mcg/ml | PO, oral; fasting; | The Pharmacological Basis of Therapeutics | C Max | 16400.0 | ng/ml | 16.4±1.2 | mcg/ml | intravenous injection, IV; | The Pharmacological Basis of Therapeutics |
| T Max | 4.0 | h | 4.0 | h | PO, oral; | The Pharmacological Basis of Therapeutics |
| Metabolic | 0 | % | 0 | % | DRUGBANK | |
| Clearance | 0.10 | L/h/kg | 1.67±0.24 | ml/min/kg | The Pharmacological Basis of Therapeutics | Clearance | 0.0900 | L/h/kg | 1.5 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 1.5 | L/kg | 1.5±0.1 | L/kg | Apparent volume of distribution; | The Pharmacological Basis of Therapeutics | Volume of Distribution | 1.2 | L/kg | 1.2 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 9.0 | h | 6-12 | h | DRUGBANK | Half-life | 10.6 | h | 10.6±1.5 | h | The Pharmacological Basis of Therapeutics | Half-life | 9.4 | h | 9.4 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 808.0 | mg/kg | 808.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 808.0 | mg/kg | 808.0 | mg/kg | PO, oral; mouse; | T3DB |
| Eliminate Route | 58.0 | % | 58±8 | % | Urinary excretion; Unchanged drug; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 43.5 | % | 20-67 | % | DRUGBANK | Protein Binding | 65.0 | % | 65±3 | % | The Pharmacological Basis of Therapeutics |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 50.0 | mg/kg/day | 50 | mg/kg/day | PO, oral | Tetracycline Hydrochloride Capsules | tetracycline hydrochloride | PDR |
| Max dose for children | 2000.0 | mg/day | 2 | g/day | PO, oral | Tetracycline Hydrochloride Capsules | tetracycline hydrochloride | PDR |
| Max dose for adolescents | 50.0 | mg/kg/day | 50 | mg/kg/day | PO, oral | Tetracycline Hydrochloride Capsules | tetracycline hydrochloride | PDR |
| Max dose for adolescents | 2000.0 | mg/day | 2 | g/day | PO, oral | Tetracycline Hydrochloride Capsules | tetracycline hydrochloride | PDR |
| Max dose for adults | 2000.0 | mg/day | 2 | g/day | PO, oral | Tetracycline Hydrochloride Capsules | tetracycline hydrochloride | PDR |
| Max dose for adults | 4000.0 | mg/day | 4 | g/day | PO, oral | Tetracycline Hydrochloride Capsules | tetracycline hydrochloride | PDR |
| Max dose for geriatric | 2000.0 | mg/day | 2 | g/day | PO, oral | Tetracycline Hydrochloride Capsules | tetracycline hydrochloride | PDR |
| Max dose for geriatric | 4000.0 | mg/day | 4 | g/day | PO, oral | Tetracycline Hydrochloride Capsules | tetracycline hydrochloride | PDR |