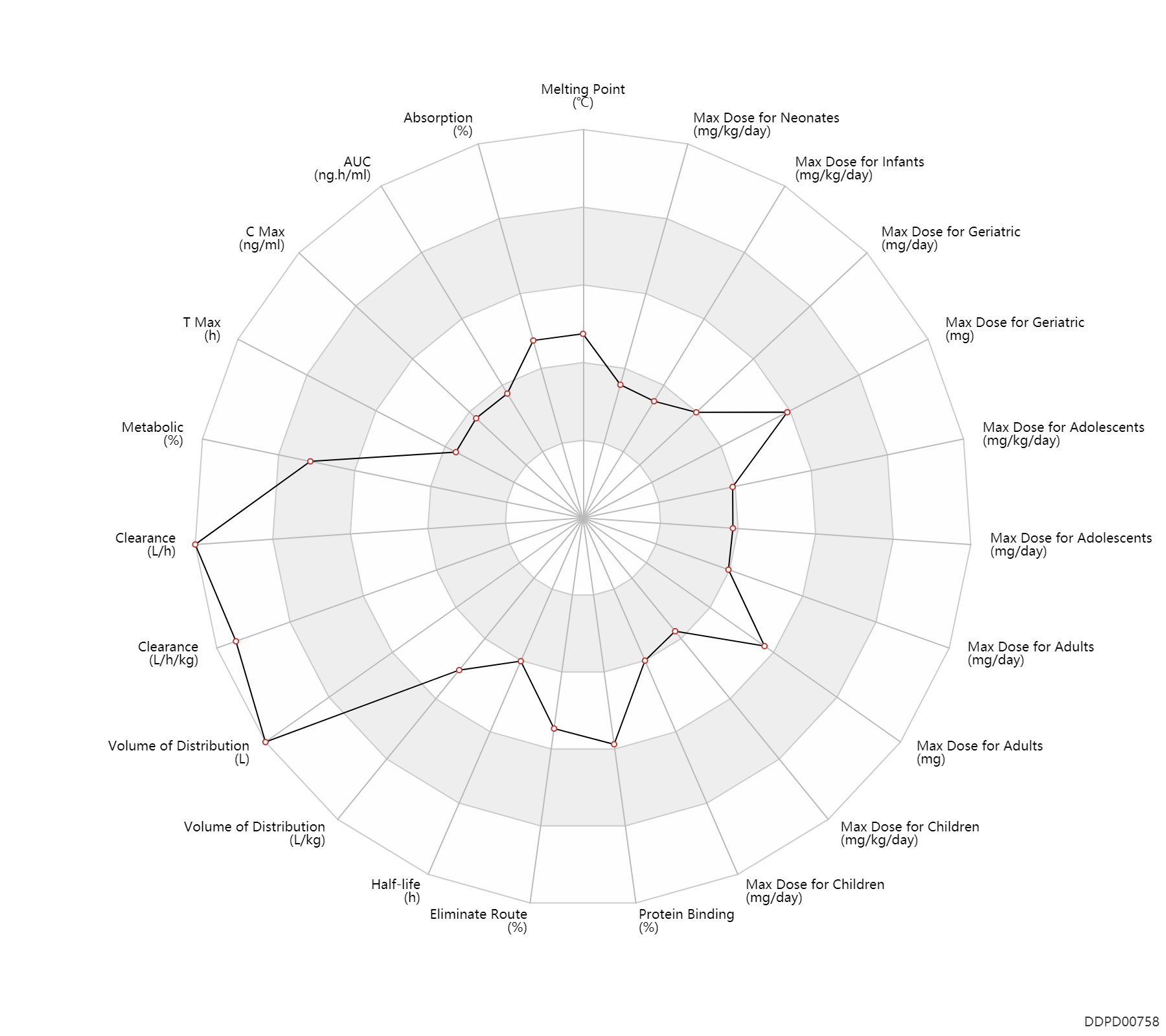
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| Absorption |
50.0 |
% |
50 |
% |
PO, oral; |
DRUGBANK |
| AUC |
45.1 |
ng.h/ml |
45.1±16.2 |
ng.h/ml |
PO, oral; poor metabolizers, PM; |
DRUGBANK |
AUC |
104.3 |
ng.h/ml |
104.3±57.3 |
ng.h/ml |
PO, oral; high-fat meal; |
DRUGBANK |
| C Max |
2.0 |
ng/ml |
2.01±2.0 |
ng/ml |
PO, oral; |
DRUGBANK |
C Max |
31.3 |
ng/ml |
31.3±13 |
ng/ml |
PO, oral; poor metabolizers, PM; |
DRUGBANK |
C Max |
60.8 |
ng/ml |
60.8±34.3 |
ng/ml |
PO, oral; high-fat meal; |
DRUGBANK |
| T Max |
0.75 |
h |
0.5-1 |
h |
PO, oral; Active metabolite; |
DRUGBANK |
T Max |
1.4 |
h |
1.40±1.07 |
h |
PO, oral; |
DRUGBANK |
| Metabolic |
85.0 |
% |
80-90 |
% |
Liver metabolism; PO, oral; Inactive metabolite; |
DRUGBANK |
| Clearance |
18960.0 |
L/h |
18960±15890 |
L/h |
PO, oral; |
DRUGBANK |
Clearance |
16980.0 |
L/h |
16980±10410 |
L/h |
PO, oral; |
DRUGBANK |
Clearance |
1.4 |
L/h/kg |
23.65 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
39240.0 |
L |
39240±33520 |
L |
Apparent volume of distribution; |
DRUGBANK |
Volume of Distribution |
2.0 |
L/kg |
2.01 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
6.0 |
h |
~6 |
h |
PO, oral; |
DRUGBANK |
Half-life |
0.50 |
h |
~30 |
min |
Active metabolite; |
DRUGBANK |
Half-life |
5.1 |
h |
5.06 |
h |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Toxicity Lethal Dose |
1750.0 |
mg/kg |
1500-2000 |
mg/kg |
Single dose; mouse; Rattus, Rat; |
DRUGBANK |
Toxicity Lethal Dose |
3000.0 |
mg/kg |
3000.0 |
mg/kg |
Single dose; non-human primate; |
DRUGBANK |
| Eliminate Route |
50.0 |
% |
50 |
% |
Urinary excretion; PO, oral; |
DRUGBANK |
Eliminate Route |
46.0 |
% |
46 |
% |
Faeces excretion; PO, oral; |
DRUGBANK |
| Protein Binding |
98.0 |
% |
98 |
% |
plasma proteins; |
DRUGBANK |
Protein Binding |
78.3 |
% |
71-85.5 |
% |
cow; |
DRUGBANK |