Basic Information
| Drug ID | DDPD00751 |

|
| Drug Name | Epinastine | |
| Molecular Weight | 249.3104 | |
| Molecular Formula | C16H15N3 | |
| CAS Number | 80012-43-7 | |
| SMILES | NC1=NCC2N1C1=CC=CC=C1CC1=CC=CC=C21 | |
| External Links | ||
| DRUGBANK | DB00751 | |
| PubChem Compound | 3241 | |
| PDR | 2150 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
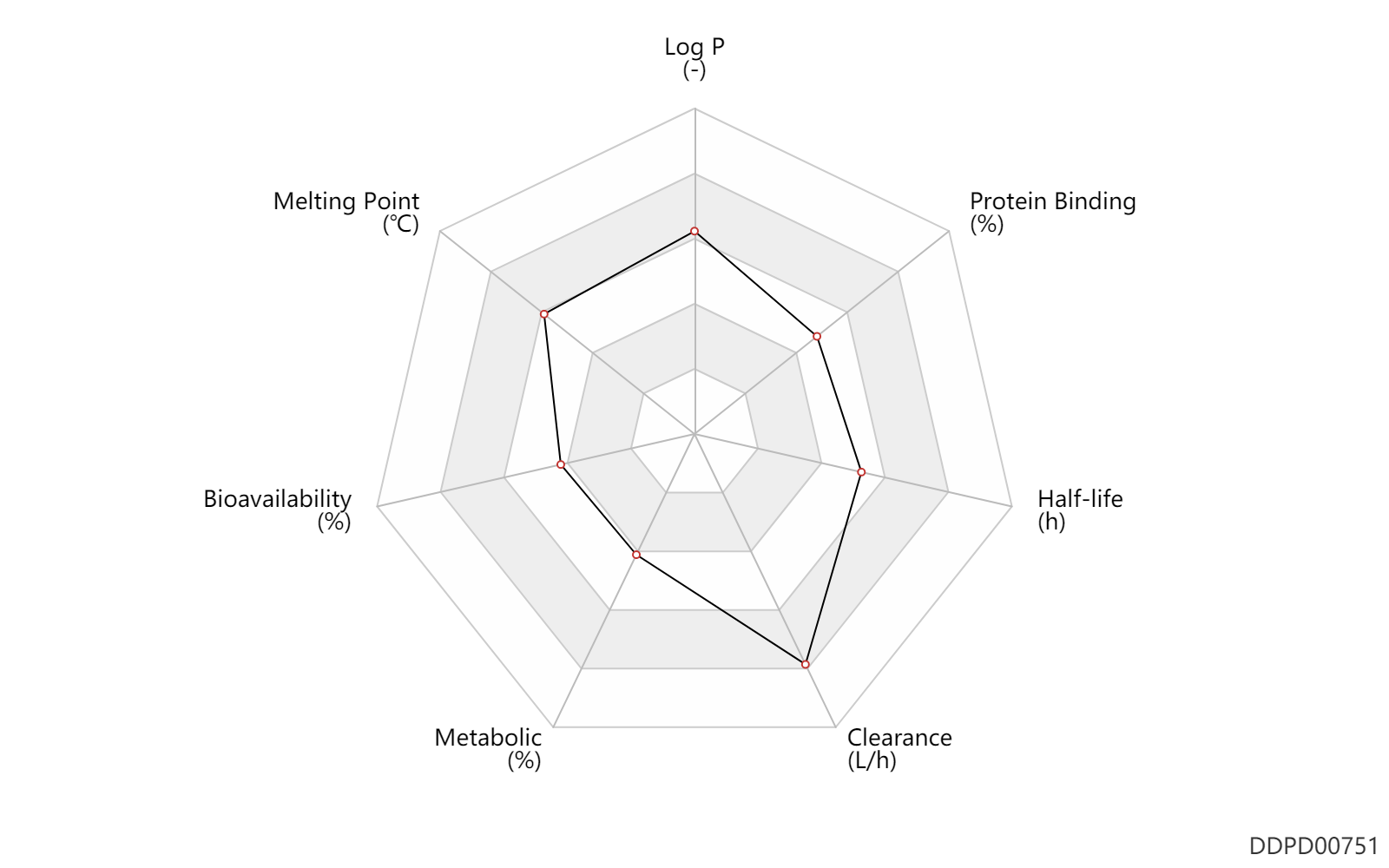
| Log P | 3.51 | - | 3.51 | - | BIOBYTE (1995) |
| Melting Point | 206.5 | ℃ | 205-208 | ℃ | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 40.0 | % | 40 | % | DRUGBANK | |
| Metabolic | 10.0 | % | <10 | % | DRUGBANK | |
| Clearance | 56.0 | L/h | 56.0 | L/h | allergic conjunctivitis; patients; ophthalmic administration; | DRUGBANK |
| Half-life | 12.0 | h | 12 | h | DRUGBANK | |
| Protein Binding | 64.0 | % | 64 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Elestat | epinastine hydrochloride | PDR |
| Max dose for adolescents | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Elestat | epinastine hydrochloride | PDR |
| Max dose for adults | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Elestat | epinastine hydrochloride | PDR |
| Max dose for geriatric | 2.0 | drop/day | 2 | drop/day | ophthalmic administration | Elestat | epinastine hydrochloride | PDR |